Once-A-Month Medication for Heroin Addiction?
Download PDF Version What is PDF?
Kimberly R. Martin
Kimberly R. Martin is a Contributing Writer for NIDA NOTES.
Source: NIDA NOTES, Vol. 19, No. 3, September, 2004
Public Domain
Table of Contents (TOC)
Article: Once-A-Month Medication for Heroin Addiction?References
A single injection of a new sustained-release formulation of buprenorphine substantially blocked heroin’s effects and relieved heroin craving and withdrawal symptoms for up to 6 weeks, report researchers at the Behavioral Pharmacology Research Unit at The Johns Hopkins University School of Medicine in Baltimore.
The study, the first to test sustained-release buprenorphine in human opioid addicts, affirms the promise of a formulation designed to increase patient adherence to treatment, ease the burden of visits to treatment providers, and reduce the risk of buprenorphine misuse.
Long-Lasting Buprenorphine Reduces Withdrawal Symptoms in Heroin-Dependent Patients
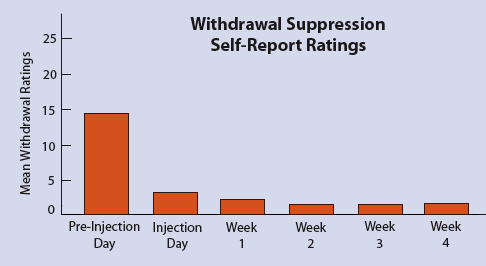 A new long-lasting, sustained-release form of buprenorphine given
by a single injection reduced patients’ heroin withdrawal
symptoms for 4 weeks after treatment.
A new long-lasting, sustained-release form of buprenorphine given
by a single injection reduced patients’ heroin withdrawal
symptoms for 4 weeks after treatment.
 Dr. George Bigelow and colleagues evaluated the formulation
with five patients, two men and three women aged 33 to 42,
who had been using heroin more than 6 years on average and
were current daily users. The day before initiating buprenorphine,
the researchers administered oral doses of hydromorphone
as clinically needed to suppress the patients’ withdrawal
symptoms. The amount of hydromorphone needed to alleviate
withdrawal symptoms is an objective measure of opioid dependence
severity. The patients’ average opioid addiction was approximately
equivalent to 50 mg/day of methadone. Buprenorphine treatment
consisted of a single injection of biodegradable polymer
microcapsules containing 58 mg of the medication. During
the following 6 weeks—a 4-week residential phase and
a 2-week outpatient phase—researchers assessed the
patients for signs of heroin withdrawal and patients rated
their withdrawal symptoms using a standard questionnaire.
No patient needed additional medication for withdrawal relief.
Dr. George Bigelow and colleagues evaluated the formulation
with five patients, two men and three women aged 33 to 42,
who had been using heroin more than 6 years on average and
were current daily users. The day before initiating buprenorphine,
the researchers administered oral doses of hydromorphone
as clinically needed to suppress the patients’ withdrawal
symptoms. The amount of hydromorphone needed to alleviate
withdrawal symptoms is an objective measure of opioid dependence
severity. The patients’ average opioid addiction was approximately
equivalent to 50 mg/day of methadone. Buprenorphine treatment
consisted of a single injection of biodegradable polymer
microcapsules containing 58 mg of the medication. During
the following 6 weeks—a 4-week residential phase and
a 2-week outpatient phase—researchers assessed the
patients for signs of heroin withdrawal and patients rated
their withdrawal symptoms using a standard questionnaire.
No patient needed additional medication for withdrawal relief.
To test sustained-release buprenorphine’s power to block the effects of heroin-like opioids, patients received weekly challenge test injections of 3 mg hydromorphone or saline under double-blind procedures. Patients’ subjective ratings of various hydromorphone effects—such as feeling high, sick, or any effect—stood at zero in the first 2 weeks after buprenorphine treatment. Drug effect ratings in subsequent weeks of the study remained low—less than 25 on a 100-point scale. Moreover, the buprenorphine formulation appeared to be safe and well tolerated, with no significant side effects or signs of opioid intoxication or respiratory depression. These results suggest that sustained-release buprenorphine may prove an appealing and effective treatment option for opioid-addicted patients and their physicians.
Sobel, B.F.; Sigmon, S.C.; Walsh, S.L.; Johnson, R.E.; Liebson, I.A.; Nuwayser, E.S.; Kerrigan, J.H.; and Bigelow, G.E. Open-label trial of an injection depot formulation of buprenorphine in opioid detoxification. Drug and Alcohol Dependence 73(1):11-22, 2004.


