Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 12
Page 122Approximately 3–5 mm
Approximately 26 ± 1 postovulatory days
Characteristic feature: 21–29 pairs of somites
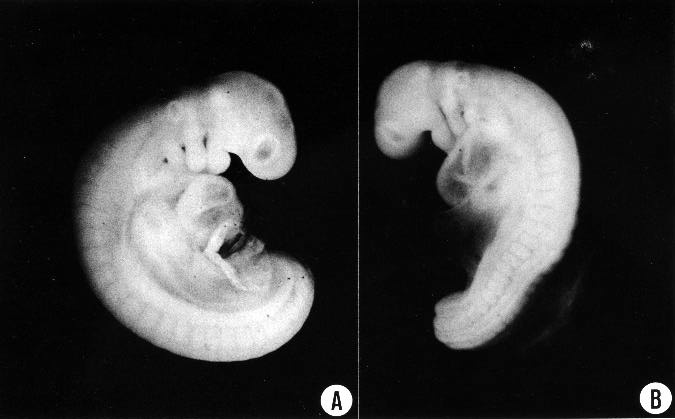
Fig. 12-1. (A) Right lateral view of No. 5923 (28 somites). See also fig. 12-2E. The internal structure is shown in figs. 12-4, 12-6, and 12-9.
(B) Left lateral view of No. 6097 (25 somites). From somite 14 caudalward the body is smaller and less mature. Hence the contour is deflected. See also fig. 12-2B.
Page 123SUMMARY
External: three pharyngeal arches are visible; the dorsal curvature of the body is becoming filled out into a smooth convexity; the caudal neuropore is closing or closed; the otic vesicles are almost closed but not detached; the upper limb buds are appearing.
Internal: the interventricular septum has begun; the cystic primordium and the dorsal pancreas are becoming distinguishable; the lung bud appears.
SIZE AND AGE
The greatest length of the specimens of stage 12 is conditioned by the curved posture that is common among them, caused by the rounding out of the thoracolumbar region. In the preceding stage the specimens were characterized by a relatively straight linear axis. The curved axis that is characteristic of stage 12 reduces their length to that extent. The result is that of seventeen specimens, measured in 80 percent alcohol, all but two have a greatest length of 3–4 mm inclusive. The two exceptions were artificially elongated and measured 4.5 and 5.3 mm, respectively. When corrected for posture, the length of each is 4.1 mm. Nine of the seventeen specimens fall between 3.2 and 3.8 mm. Thus the length of the embryo at this time is misleading as an indication of growth, because these measurements are about the same as those of the previous stage.
As for the size of the chorion, one must omit tubal specimens, which are usually undersized. Moreover, some uterine specimens are too large. In the latter cases the chorion appears to have grown after arrest of development of the embryo. Not counting the tubal specimens, out of ten examples studied in which the size of the chorion was recorded, eight had a greatest diameter of 20–25 mm. Two were over 30 mm. If the mean of the greatest and least diameters be taken as the average diameter, eight of the ten specimens showed an average diameter of 15–20 mm, whereas two had average diameters of 20–25 mm. The age of embryos of stage 12 is believed to be approximately 26 postovulatory days.
EXTERNAL FORM
When the number of somites in a given embryo is known, it can be decided immediately whether or not it belongs to this stage. The number is not known precisely, however, until the embryo has been cleared or has been sectioned serially. Furthermore, it should be kept in mind that, from 10 somitic pairs to the end of stage 11, somite 1 is generally much smaller than somite 2. In stages 12 and 13, somite 1 is contributing to the hypoglossal cord (O'Rahilly and Müller, 1984b) and hence, “in embryos with more than 20 somites” (i.e., from the beginning of stage 12), the first ones visible “actually are second somites” (Arey, 1938).
Four occipital somites are present, as determined by their relationships to (1) the concentrations of the cervical neural crest (which are caudal to the occipital region), and (2) the primordia of the hypoglossal nerve.
An apparent rotation of the somites takes place between stages 11 and 12 (O'Rahilly and Müller, 1984b). The longitudinal axis of the dermatomyotome, as seen in cross section, comes to make a more acute angle with the median plane. Associated with this, the dorsal surface of the body, as seen in cross section, is changing from a gentle to a steeper curvature.
Among the several features that are characteristic, a prominenc one is the presence of three pharyngeal arches, in contrast with the two of the preceding stage. There are now three bars and three membranes where skin and gut epithelia come in contact, and furthermore Page 124 the pharyngeal arches are subdivided into dorsal and ventral parts. In exceptional cases in later stages, a fourth pharyngeal arch may appear, but three are the usual final complement seen externally. Caudal to the third there is a depression consisting of the condensed mass that is to form the mesoblastic elements of the larynx, and it is in this mass that the superior laryngeal nerve terminates. This is the region of the cervical sinus, which will be considered with the next two stages.
The previous stage was characterized by successive steps in the closure of the rostral neuropore. Stage 12 is similarly marked by the gradual closing of the caudal neuropore, which process is completed in the more advanced members of the group. With the increase in bulk of the spinal cord, somites, and surrounding mesoblast, the back of the embryo becomes filled out and takes a characteristic C-shaped form, eliminating the easily flexed region at the levels of somites 10–18, which so commonly results in a dorsal kink in stage 11.
Other features defining the group include the near closure of the otocyst, a slight opening or pore being still recognizable in the more advanced members in at least one of the vesicles. Also, what had been a wide opening between gut and umbilical vesicle now begins to narrow down so that an umbilical stalk is taking form. Finally, in distinction from the next stage, there is no really conspicuous upper limb bud. In the less advanced embryos none can be seen. In more-advanced specimens a condensation representing the primordium of the upper limb bud can be fairly well outlined. It is centered opposite somites 8–10 and merges caudally with the lateral unsegmented strip of mesoblast that is to form the ventrolateral body wall.
The ectodermal ring, described by Schmitt in 1898 and named by Blechschmidt in 1948, is complete at stage 12 (O'Rahilly and Müller, 1985). The ring, which may well be an important example of epithelial-mesenchymal interaction, comprises six parts: (1) the rostral part, containing the situs neuroporicus, and nasal and lens discs, (2) the pharyngeal part, the covering of the pharyngeal arches, (3) the occipital and cervicothoracic parts, related at first to the four occipital somites and later to the cervicothoracic junction, (4) the membral part, represented by a preliminary ectodermal thickening, followed within 2 days by the apical ectodermal ridge, (5) the intermembral part, related at first to the underlying coelom, and mesonephric duct and ridge, and (6) the caudal part, containing the cloacal membrane and a temporary “caudal ectodermal ridge.” It is stressed that the incorrectly named Milchstreifen is merely the intermembral part, in which the mammary crest (Milchlinie or Milchleiste) appears one week later.
CARDIOVASCULAR SYSTEM
In stage 11 the coelom provides a means by which the exocoelomic fluid is brought into direct contact with the deeply lying mesoblast. By such a system of irrigation the increasing amount of embryonic tissue is insured an adequate supply of nutriment. At its best, the coelom provides only an elementary type of circulation. It is certainly an improvement on the tissue culture mechanisms of the presomitic period, but it will not prove adequate for the larger and more elaborate organism that is to follow. Already in stage 12, the principal solution of this problem, in the form of a blood vascular system, is well under way.
Page 125
Fig. 12-2. (A) Dorsal view of No. 7852 (25 somites). The fourth ventricle is evident and the trigeminal ganglia can be distinguished. See also fig. 12-2C.
(B) Ventral view of No. 6097 (25 somites). The remains of the oropharyngeal membrane can be seen. The right and left mandibular arches are partially united in front. See also fig. 12-1B.
(C) Left lateral view of No. 7852 (25 somites). The otocyst, trigeminal ganglion, and vestibulofacial complex are distinguishable. The cardiac and hepatic regions are translucent. See also fig. 12-2A.
(D) Left ventrolateral view of No. 6488 (28 somites), showing junction of umbilical vesicle and intestine. Here one can speak of an umbilical stalk. Note the free opening to the coelom.
(E) Ventral view of No. 5923 (28 somites), showing the umbilical vesicle. The neural walls are visible in the head, and the optic vesicles can be distinguished. See also fig. 12-1 A.
Page 126
Fig. 12-3. Profile reconstruction of the digestive system in a 29-somite embryo (No. 1062), based on a photograph. Several of the primary endodermal derivatives are sharply delimited, and their topography relative to the body of the embryo is indicated. The omental bursa is shown because this marks the region that is to form the stomach. In reality the bursa lies to the right of the gut, as shown in section C, figure 12-4. The urinary system included in the figure was drawn from a plaster reconstruction.
The coelomic channel is part and parcel of the mesoblast, and as late as 30 somites is still meeting most of the circulatory requirements of the mesoblastic derivatives. The brain and spinal cord, however, which together constitute the largest and most compact tissue mass in embryos of stage 12, do not share in its services. Earlier the neural tissue, in the form of a plate, is exposed directly to amniotic fluid, which at that time appears to differ but little from the general exocoelornic fluid. The proliferating neuroectodermal cells are thereby just as well supplied as the cells of the skin ectoderm. But when the neural plate becomes the neural tube and becomes separated from the surface by the overlying skin ectoderm, its cells are deprived of their previous source of nutrient fluid. Their protoplasmic requirements call for some new provision, and this is supplied by the blood vascular system. As soon as the margins of the neural folds begin to fuse across the median plane, which occurs during stage 10, one finds endothelial sprouts extending dorsally from the aortae toward the neural tissue. These sprouts are found in the spaces that intervene between the somites, and are designated the dorsal segmental arteries, The sprouts soon anastomose and spread as a capillary plexus, closely investing the surface of the neural tube.
At the same time, superficial to and connected with the capillary sheet of the neural tube, a loose plexus is laid down in the mesoblast lateral to the neural tube, including the brain. It is evident that throughout the early mesoblast there are many cells having the potentiality of becoming blood capillaries, and needing only an appropriate stimulus. In the plexus that is forming about the brain and spinal cord, one cannot always be sure whether a given vessel is a sprout from the aortic branches or a new structure differentiated in loco. Judging from the detached way in which its units make their appearance, it is probable that the superficial lateral plexus here referred to is differentiated in loco. The early steps in the formation of this plexus can be seen in stage 11, as shown schematically in figure 11-7. The more advanced phases are in the cranial region, and are particularly associated with the cranial ganglia and neural crest cells, which may well be the stimulus to endothelial differentiation. Main channels soon make their appearance so that one can speak of a primary head vein, and along the spinal cord one can soon recognize the cardinal veins. In stage 12 the rostral and caudal cardinal veins flow together bilaterally as the right and left common cardinal veins, which in turn empty into the sinus venosus. By that time the blood flow has a definite direction, and the central nervous system can be said to have at least a surface blood supply and drainage. In this respect it is somewhat in advance of any other of the permanent organs.
Page 127
Fig. 12-4. Profile reconstructions of the digestive system in three embryos (No. 5056, 25 somites; No. 6097, 25 somites; and No 5923, 28 somites). All three are based on sections enlarged to the same scale A–F, detailed sections of the 28-somite specimen. For their position in the embryo these drawings can be compared with figure 12-3. The sections show the degree of specialization of the gut epithelium at six levels. In sections C and F, similar specializations are present in the coelomic wall, the cells of which will compose the connective tissue, muscle, and blood vessels that are to combine with the epithelium to constitute the wall and mesenteries of the gut.The residual coelomic surface cells will form the peritoneum, which is antedated by the omental bursa.
Page 128
Fig. 12-5. (A) Section through the hepatic region of a 25-somite embryo (No. 7852), showing the invasion of the stroma of the liver by the hepatic epithelium. (B) Part of the same section.
In embryos of 14 paired somites the blood circulation is largely limited to an ebb and flow in the wall of the umbilical vesicle, produced by the early pulsations of the heart. In general, the flow is toward the heart from the rostral region of the umbilical vesicle, and toward the caudal part of the umbilical vesicle from the aorta. Annexed to this system is the connecting stalk. This is primarily associated with the allantoic diverticulum, and its large vessels are to be converted into the umbilical veins and arteries. These vessels will constitute the highway to the stroma of the placental villi, the stroma being separated from the maternal blood by the villous epithelium. Such is the picture in stage 11. In stage 12 one encounters several modifications and adaptations that produce for the first time a simple but connected circulatory system. A profile reconstruction of the larger elements of the vascular system of one of the more advanced embryos is shown in figure 12-6, and this should be compared with figure 11-7 of the preceding stage. A list of the principal items in vascular specialization found at this stage would include: (1) changes at the venous end of the heart, (2) vascularization of the central nervous system, (3) establishment of the cardinal venous drainage, (4) the hepatic plexus, and (5) alterations in the vitelline plexus resulting in main trunks, representing vitelline arteries and veins.
Page 129
Fig. 12-6. Profile reconstruction of the blood vascular system in a 28-somite embryo (No. 5923). The liver, the umbilical vesicle, the cranial ganglia, and the surface of the central nervous system have a good blood supply, whereas the caudal end of the embryo is less advanced. The plexiform pattern is characteristic of rapid transformations and provides the means for caudal migration of the communications of the umbilical arteries and veins. The smaller capillaries are not shown, although they can be seen in the sections.
The changes at the venous end of the heart during stages 10–13 are shown in figure 12-7. One can see how enlarged parts of the vitelline plexus become the right and left atria, and how on each side an enlarged sac of the plexus becomes the point of outlet for the common cardinal vein. As part of the latter transformation, the left atrium becomes cut off from the channel that is to form the sinus venosus. The sinus venosus at this stage serves as a terminal venous reservoir into which blood from all parts of the embryo is emptied. It is separated from the right atrium by a narrow passage, the sinu-atrial foramen, which, reinforced by the enclosing myocardium and gelatinous cushions, appears to have the effect of hindering a backflow of blood on contraction of the atrial myocardium (fig. 12-8). In other words, it could be regarded as a provisional element in the mechanism that directs the blood flow during the period before true valves have formed. The formation of atrial chambers and the atrioventricular canal, combined with proper synchronization of the myocardium, also serves the same end. At any rate, the heart is now capable of maintaining some circulation of blood around the surface of the central nervous system, throughout the wall of the umbilical vesicle, and through the chorion and its villi. Compared with the later placenta, this circulation is elementary, and Page 130 in the early villi the interchange must be scant between the embryonic blood and the maternal blood of the intervillous space.

Fig. 12-7. Drawings made from reconstructions showing the venous end of the heart viewed from the front. The series includes four stages (10–13), and represents embryos having 10–32 somites. The collection number of the embryo and number of somites are indicated in each case. All are enlarged to the same scale. Except in the least advanced specimen, the ventricular part of the heart is removed at the atrioventricular junction. It can be seen that the atria are new formations, superimposed on the vitelline plexus. The sinus venosus retains more definitely its identity with the vitelline plexus, taking on the character of a reservoir into which all the veins of the embryo empty their contents. It, in turn, discharges its contents through the sinu-atrial foramen into the right atrium. This foramen, therefore, marks the boundary between veins and heart proper. The marked expansion of the atria in the 28- and 30-somite embryos is coincident with the specialization of the coelomic wall of the hepatocardiac channel, by which it appears to become more permeable to coelomic fluid. Compare figure 12-10. Abbreviations: A–V. JCT., atrioventricular junction; L.C.C., left common cardinal vein; R.C.C.,right common cardinal vein; L.U.V., left umbilical vein; R.U.V., right umbilical vein; LO-M.V., left omphalomesenteric vein.
The blood supply of the central nervous system and the establishment of the cardinal veins have already been mentioned. The specialization of the vessels in the hepatic region (item 4 in the above list) needs special attention. It is best seen from the right side. Already marked differences, have developed between the two sides, an asymmetry that dates back to at least stage 10, when the lower end of the ventricular region, in its elongation, first becomes deflected to the left. The asymmetry is more pronounced in the atria and sinus venosus than in most of the tributary vessels. The vessels of the hepatic region are an exception. They become right- and left-sided very early, as is shown in figure 12-7. The relationships of this region are shown in figure 12-9, which is based on a right-profile reconstruction of the embryo shown in figure 12-6. The heart proper may be said to start at the sinu-atrial foramen, whereas the sinus venosus serves as a common channel into which all the afferent veins empty. The common Page 131 cardinal and the umbilical veins terminate in about the same way on the two sides. The hepatic plexus is less symmetrical. The main part of the plexus is derived from the newly formed capillaries in the hepatic primordium. Anastomosing with the hepatic plexus on each side are the vitelline veins, which are transformed and enlarged elements of the vascular plexus of the umbilical vesicle. Dorsally the hepatic plexus empties by multiple anastomoses into an enlarged passage, the hepatocardiac channel, which leads to the sinus venosus and thereby to the heart. This channel is either single or multiple. It reaches its full expression on the right side (fig. 12-9). The tributary channel from the left side is more plexiform, and its communication with the sinus venosus is transitory. By virtue of the plexus and these channels, the precocious primordium of the liver is highly vascularized and is in free communication with the plexus of the umbilical vesicle on the one hand and the heart on the other. This is in contrast with the remainder of the digestive system, which thus far shows scant evidence of activity.
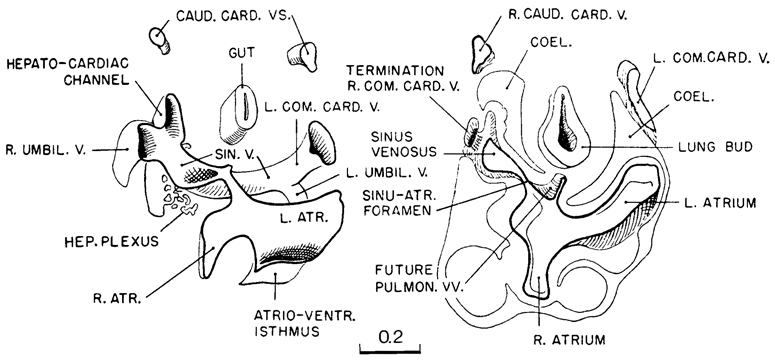
Fig. 12-8. Simplified drawings to show the communications at the venous end of the heart in a 28-somite embryo (No. 5923). The drawings represent two slabs. The caudal one (left) shows the relations of the sinus venosus beyond the level of the atria. The other slab is to be thought of as fitting directly over the caudal one. It shows the top of the sinus venosus and its communications through the sinu-atrial foramen with the right atrium. In this slab the coelomic contours are shown by a thin line. One can see the area of specialized coelomic wall over the hepatocardiac channel and the communication of the latter with the sinus venosus. The device would appear to facilitate the passage of coelomic fluid into the blood stream. Compare with figure 12-10. These sketches were drawn from a transparent model of the heart made by Osborne O. Heard. Tracings of the sections were made and oriented on transparent films (cellulose acetate) and the whole series superimposed for study with illumination coming from below. The heart of this embryo was reconstructed anew by de Vries and Saunders (1962, plate 5).
A special feature that characterizes the right hepatocardiac channel, and to a lesser extent the left, is a specialization of the coelomic wall, illustrated in figure 12-10. It is a provision by which a section of the large hepatocardiac channel is immersed in the coelomic channel. It is separated from the coelomic fluid by only the sharply marked-off specialized area of what is apparently easily permeable coelomic tissue. It consists of a thinned-out vesicular covering, the large spaces of which are filled with clear fluid. Embryos at this time are doubtless to some degree permeable through all surfaces, but there are some areas which, judging from their histological appearance, are more permeable than others. The areolar character of the connecting stalk indicates that it and its contained large vascular channels must be readily permeable to coelomic fluid. This condition goes back to presomitic embryos, in which the foamy character of the connecting stalk was pointed out and illustrated by Heuser (1932b). The specialized area, where the large hepatocardiac channel projects into the coelom, appears to be even more favorable than the connecting stalk to the give and take between the coelomic fluid and the blood stream. It would seem that it is this kind of mechanism that provides the large amount of plasma necessary to fill the endothelial system, which at this time is so rapidly enlarging. This finding is supported by the fact that the filling out and expansion of the right and left atria follow promptly the differentiation of this particular coelomic area. Later, when the villous circulation becomes efficient and interchange is inaugurated between embryonic and maternal blood, the need for this device will have passed.
Page 132
Fig. 12-9. Profile reconstructions of the right side of a 28-somite embryo (No. 5923). From this view one can see the formation of the hepatocardiac channel and its termination in the sinus venosus, and also the termination of the latter in the right atrium. It is over the rostral part of the hepatocardiac channel that the coelomic wall is structurally modified with respect to permeability, apparently facilitating exchange between the coelomic fluid and the blood stream. Compare figure 12-10.
Page 133
Fig. 12-10. Four sections at the same scale, showing the specialization of the coelomic wall over the large venous channels opening into the sinus venosus. These are from four different embryos and represent four different degrees of specialization. The effect appears to be an increase of permeability, facilitating exchange of fluid between the coelomic cavity and the bloodstream at the strategic point just where the blood enters the heart.
(A) 28-somite embryo (No. 7999, section 2-5-7), showing right hepatocardiac channel opening (below) into sinus venosus. Where the channel projects into the coelomic passage (above), the wall is specialized as a fluid-filled reticular tissue. Droplets of fluid can be seen in the coelomic surface cells. The medial coelomic wall is characterized by a thick zone of proliferating cells that are becoming differentiated and detached to form the mesoblastic wall and enveloping tissues of the gut epithelium, the edge of which can be seen on the right side.
(B) Left hepatocardiac channel of a 25-somite embryo (No. 7852, section 2-3-4). The coelomic wall is less advanced in its specialization than in A.
(C) Section through the right hepatocardiac channel of a 28-somite embryo (No. 5923, section 2-1-9). The channel opens widely into the sinus venosus, essentially like that in A. Asterisk: coelomic cavity.
(D) Left hepatocardiac channel in a 25-somite embryo (No. 6097, section 2-3-8). As is common on the left side, this channel is plexiform. The coelomic wall is the most advanced in its specialization of the four examples shown here.
Page 134In stage 12 the right venous valve and the atrioventricular canal develop, and septum primum and foramen primum may appear (McBride, Moore, and Hutchins, 1981).
The circulatory system, which is now connected for the first time, comprises the following sequence: umbilical (and hepatocardiac and common cardinal) veins, sinus venosus, sinu-atrial foramen, right atrium, left atrium, atrioventricular canal, left ventricle, right ventricle, conus cordis, truncus arteriosus, aortic arches, aorta, and umbilical arteries.
The four main portions of the primary cardiac tube are the trabeculated part of the left ventricle, the trabeculated part of the right ventricle, the conus cordis, and the truncus arteriosus (O'Rahilly, 1971, in whose table 1 synonyms are given). The last two segments compose the outflow tract. These features are well seen in the heart illustrated by Rosenbauer (1955).
A historical review of the nomenclature of the embryonic heart has been published, but the conclusion that the term truncus arteriosus be used for the entire “arterial pole” from the ventricle to the origin of the aortic arches (Laane, 1974) seems to be highly inadvisable and is not followed here.
DIGESTIVE AND RESPIRATORY SYSTEMS
One of the major differences between stages 11 and 12 is the marked advance in the differentiation of the alimentary epithelium in the latter. Sharply outlined fields of epithelial proliferative activity mark the location of such primary organs as liver, lung, stomach, and dorsal pancreas. This occurs with such uniformity in the various members of the group that aside from their other characteristics it serves as a useful test of their eligibility for the group.
The position of the gut in relation to the embryo as a whole is shown in figure 12-3. This has been plotted for one of the more advanced embryos (29 somites). The digestive systems of three other embryos (25, 25, and 28 somites) are shown in figure 12-4. These plots are based on profile reconstructions, and show topographically the distribution and relative sizes of the early centers of epithelial proliferation.
In the pharyngeal region the more active proliferation is in the floor, in contrast with the roof, which is relatively thin. Similarly, the alimentary epithelium is thin as compared with that of the respiratory diverticulum. Among the earliest of the pharyngeal proliferative centers is the unilateral median thyroid, which projects ventrally into the concavity of the truncus arteriosus, and the telopharyngeal bodies, which proliferate bilaterally to constitute, according to Weller (1933), the lateral components of the thyroid and parathyroid glands. These particular fields of pharyngeal epithelium are already specialized and definitive, and thus far no environmental stimulus has been identified as a cause of their differentiation. Until such a stimulus is discovered we must conclude that this primary proliferation is inherent in the genic constitution of the cells. It is subsequent to this that we find the environment playing a part in the development of these structures. That the components of the thyroid are so precocious is an indication of the basic importance of this gland in development and growth.
Similarly, the greater proliferation shown by the respiratory as compared to the adjacent alimentary epithelium would seem not to be environmental in origin. Histological pictures of sections through the level of the lung bud are shown in figure 12-4A, B. These are adjacent sections and they illustrate the greater precocity of the respiratory epithelium as compared with that of the adjacent alimentary canal, which forms the dorsal half of the digestive tube. The only difference in environment, ventrally and dorsally, is the presence of a few capillaries from the sinus venosus which at this time extend toward the pulmonary evagination and are the precursors of the pulmonary veins (compare fig. 12-8). It would appear likely that the capillaries are a response to the pulmonary evagination rather Page 135 than the reverse, in view of the sensitivity of capillaries to stimuli. The sections A and B indicate a specialized pulmonary epithelium.
A section immediately below the level of the lung bud is shown in figure 12-4C. The presence of the beginning omental bursa marks this part of the gut as the location of the stomach. It is at this time, between 25 and 28 pairs of somites, that the gastric part of the canal becomes elongated, increasing the distance between the lung bud and the hepatic diverticulum. It is not until the next stage that it undergoes the fusiform enlargement that is characteristic of the stomach. A transverse section through the gastric region (fig. 12-4C) reveals a very active proliferation of the coelomic cells, which are moving in to form, in course of time, the connective tissue coats and muscular wall of the stomach together with its rich supply of blood vessels.
Caudal to the stomach, one encounters an area of proliferation in the dorsal half of the intestinal epithelium that is to become the dorsal pancreas. This is sharply marked off from the adjacent intestinal wall, as can be seen in section D, figure 12-4. A little more caudally and on the opposite side is the hepatic diverticulum, which because of its special precocity will be described in a separate section. At the level where the gut opens into the umbilical vesicle, one can recognize a sharp transition from intestinal epithelium to the inner layer of the umbilical vesicle, although the two are continuous. This is true also of the enclosing coelomic tissues. By comparing 25-somite specimens with those having 28–30 somites, it will be seen that the opening between gut and umbilical vesicle becomes constricted. This constriction comprises not only a craniocaudal flattening but also an actual decrease in size of cross section. With this, there is a corresponding elongation of the umbilical vesicle, and in the 30-somite specimen one can speak of a vitelline duct, which is associated with the diverticulum ilei. In this way, a landmark indicates the junction of foregut and hindgut, cranial to which all of the small intestine is derived save the terminal third of the ileum, and caudal to which the remaining third of the ileum and the large intestine take origin. Certainty for this landmark, however, would require ruling out the occurrence of caudal migration of the point of attachment of the vitelline duct. The hindgut during this period is in an elementary state of organization, particularly toward the caudal end. Comparison of the reconstructions shown in figure 12-4 reveals an elongation of the interval between the junction of the umbilical vesicle and the junction with the allantoic duct.
Additional features of the digestive and respiratory systems at stage 12 include the following: remains of the ruptured oropharyngeal membrane, the cervical sinus caudal to pharyngeal arch 3, the telopharyngeal body being separated from the caudal pharyngeal complex (pouches 3 and 4), and the tracheo-esophageal septum (O'Rahilly and Müller, 1984c), but no common esophagotrachea.
Liver
Of the different organs that compose the digestive system, the liver is by far the most precocious. Whether this is because it lies at an active center of angiogenesis or whether the development of the liver is the cause of the surrounding vascular activity is an open question. In the preceding stage it was seen that proliferation of the hepatic epithelium was accompanied by the formation of blood islands among the adjacent cells, evidently stimulated by the epithelium. In general, the embryonic endothelial apparatus is responsive to the stimulation (i.e., the requirements) of the surrounding structures. Hence it seems likely that the precocity of the liver is caused by the inherent constitution of its component cells. In consequence, the liver becomes established as a functioning organ early in development.
It has been shown in stage 11 that the stroma, or framework, of the liver is derived from proliferating mesoblastic cells that become detached from the surface germinal bed of the coelom in the cardiac region, whereas the parenchyma of the liver is derived from a specific area of alimentary epithelium that shapes itself as the hepatic diverticulum. The advance that is characteristic of stage 12 consists in the spread of the epithelium of the diverticulum outward into the stroma as a fringe of epithelial trabeculae, as is shown in figure 12-5. It will be seen that the digestive epithelium, because of its irregular foci of proliferation, loses the smooth contour of its outer surface and merges into the columnar extensions. In making this invasion the epithelial trabeculae enmesh the newly formed stromal capillaries. New stimulation is thereby given Page 136 to angiogenesis, and a large part of the stroma is converted into capillaries and blood cells. A relatively small part is required for capsule and interlobular connective tissue. In the less advanced members of the stage the epithelial invasion is just starting, and in the more advanced members it has spread through about one-half of the hepatic primordium. The invasion iscompleted in stage 13.

Fig. 12-11. Three phases in the development of the otocyst during stage 12. (A) 25-somite embryo (No. 6097, section 1-3-1). (B) 25-somite embryo (No. 7852, section 1-2-9). (C) 28-somite embryo (No. 5923, section 1-2-11). The ectoderm of the otocyst resembles that of the brain wall and is highly specialized compared with the simpler skin ectoderm, from which it is sharply demarcated. The three drawings are enlarged to the same scale.
The caudal part of the hepatic diverticulum becomes set off from the start, forming a subdivision that constitutes the primordium of the cystic duct and gall bladder. This part does not participate in the formation of epithelial columns or in the invasion of the hepatic stroma. A typical section through this region is shown in figure 12-4, section E. Thus, this area of digestive epithelium is already specialized, having been assigned to the liver, and the part that will form hepatic cells and biliary ducts has already been distinguished from the parts that will form gall bladder and cystic duct.
Intervening between the liver and the heart is a conspicuous plexus of blood capillaries. During this period these vessels are rapidly becoming enmeshed by the proliferating hepatic epithelium, and it is quite likely that they are a response to its presence. The more ventrally lying capillaries are differentiated in situ in the primordial stroma of the liver. The larger dorsal channels are enlargements and anastomosing extensions of the network of the umbilical vesicle. It is not accurate, however, to speak of the latter as the omphalomesenteric veins, which will make their appearance later as derivatives of part of the umbilical plexus. During the period under consideration these venous channels serve to drain the liver into the sinus venosus and through it into the venous end of the heart. A special function appears to be assigned to the large, sinus-like channels of the plexus, which occur bilaterally along its dorsal margins. For convenience these will be spoken of as the hepatocardiac channels. These passages and the mesoblastic cells surrounding them apparently correspond to what is spoken of by some writers as the right and left horns of the liver.
Two embryos of stage 12 were included in Lipp's (1952) study of the liver, and further details in staged embryos have been provided by Severn (1971, 1972).
URINARY AND REPRODUCTIVE SYSTEMS
The varied and highly specialized mesoblastic tissues that are derived from various areas of the coelom have already been noted. The mesonephros and its duct constitute another example of such specialization. In figure 12-3 is shown a profile view of the mesonephros as found in a 29-somite embryo, one of the Page 137 more advanced members of stage 12. It is slightly less advanced than the first of those described by Shikinami (1926). The tubules begin at the level of somite 8 and are distinct as far caudally as somite 20, whence they extend as a continuous nephrogenic cord to the level of somite 24. Opposite each somite there are two or more tubules. Thus they are not metameric, any more than the mesonephric duct is metameric, or the umbilical vein.
The number of nephric vesicles is being increased by progressive differentiation caudally from the nephrogenic cord (Torrey, 1954). The mesonephric duct at first ends blindly immediately short of the cloaca, but soon becomes attached to the cloaca (i.e., to the terminal part of the hindgut) and acquires a lumen.
The primordial germ cells are in the wall of the hindgut (Witschi, 1948). Details of the arrangement of the germ cells in an embryo of 26–27 pairs of somites have been provided by Politzer (1928b).
NERVOUS SYSTEM
The form of the central nervous system is shown in figure 12-6. It can be seen that the relatively large and compact neural tube is in large part responsible for the C-shaped outline that is characteristic of the embryo at this time. With the closure of the caudal neuropore, the caudal end of the tube becomes defined, and further elongation of the body is attained more by interstitial growth and less by segregation of neural ectoderm and mesoblastic somitic material, as is characteristic of the preceding stages. The part of the neural tube caudal to somite 24 is still undifferentiated and small, and conversely the hindbrain and cervical-cord regions are large and advanced in their differentiation. Perhaps the most notable improvement to be seen in the neural tube is the provision of a blood supply to its surface.
At stage 12 the first phase in the formation of the neural tube (primary neurulation) ends. Further elongation is caused by a poorly understood process termed secondary neurulation, in which neural ectoderm is not involved (Lemire et al., 1975).
The roof of the rhombencephalon is becoming thin. Eight rhombomeres can be distinguished. A sheet of motor neurons is present in the basal plate of the rhombencephalon, and is particularly clear in those embryos in which the roots of the hypoglossal nerve begin to differentiate (O'Rahilly and Müller, 1984b). Neurofibrils begin to form for the first time in the rhombencephalic wall, and in most embryos a marginal layer begins to develop. The cells constituting the superior ganglia of cranial nerves 9 and 10 are present as neural crest material.
The telencephalon enlarges and includes the embryonic lamina terminalis and the telencephalon medium rostral to the optic vesicles (fig. 13-10). The area adjacent to the closed, former rostral neuropore is the embryonic commissural plate. Thickenings in the diencephalon indicate the primordia of the ventral thalamus and the subthalamus. The diencephalic ventral thickening is continuous with a thickening in the midbrain that constitutes the tegmentum; the latter is clearly set off by the sulcus limitans, which at this time continues into the diencephalon. The mesencephalon has grown considerably and comprises two segments (M1, M2).
The nasal discs become thicker and form a portion of the ectodermal ring on both sides of the closed neuropore (O'Rahilly and Müller, 1985). The olfactory area of the brain is also becoming thicker, and mitotic figures become more numerous (Bossy, 1980a).
Eye
At stage 12 the optic neural crest reaches its maximum extent and the optic vesicle becomes covered by a complete sheath, giving the appearance of a “frightened hedgehog” (Bartelmez and Blount, 1954).
Ear
The otocyst in embryos possessing 21–29 pairs of somites is one of the most reliable characteristics for determining the degree of development. In the less advanced specimens the otocysts are conspicuously open to the surface, whereas in the more advanced members of the group the closure is nearly complete and detachment from the skin ectoderm is imminent. In the gross specimen the pore may be so small that it escapes attention and is not found until seen in sections. Three typical specimens are shown in figure 12- 11. These are selected from well-preserved embryos, Page 138 and their histology and general form can be relied upon. It is to be noted that the epithelium of the otocyst is highly specialized compared with the simpler skin ectoderm, from which it is sharply demarcated. The fact that from the outset it can be traced as a specialized area is good evidence that it is not to be regarded as modified skin, but rather as a detached island of neural ectoderm, related to but not identical with the ectoderm of the brain wall. It is to be added that in poorly preserved and in macerated embryos the otocyst may show distortions and abnormalities in form. It may be collapsed or distended. Distortions are commonly seen at the junction of the otocyst with the skin ectoderm. Thus there may be an unduly wide opening to the surface, but more frequently the open rim of the otocyst is stretched into a duct-like stalk by which the otocyst retains its attachment to the surface. This should not be confused with an endolymphatic appendage.
Vestibular neural crest is still forming from the wall of the otic pit and developing vesicle.
SPECIMENS OF STAGE 12 ALREADY DESCRIBED
22 somites, Girgis embryo, Royal School of Medicine, Cairo. Apparently a normal embryo, studied and reconstructed by the wax-plate method at the Institute of Anatomy, University College, London by Girgis (1926). Otic vesicle and caudal neuropore both still open. Three pharyngeal arches are present. Umbilical vesicle opens rather widely into gut. Morphology of central nervous system, digestive system, vascular system, and excretory system described in detail. The specimen had been kept in alcohol for a prolonged period, and apparently is not suitable for cytological minutiae.
22 somites, Carnegie No. 8963 (University of Chicago No. H 1093).Described by Wen (1928) with particular reference to the nervous system.
23 somites, Van den Broek embryo “A,” Zentral-Institut für Hirnforschung, Amsterdam.Two similar embryos are described together by Van den Broek (1911). The description refers almost entirely to “A,” which is the better preserved of the two. It is evidently normal, and judging from the form of the brain, open otocyst, and liver it corresponds to about a 23-somite embryo, although it is said to have 21–22 somites.
23 somites, R Meyer, No. 300. The Meyer collection was transferred from Berlin to the University of Zürich in 1922, and placed in charge of H. Frey. This valuable specimen was described in monographic form by Thompson (1907, 1908). Also described and figured in the Keibel and Elze Normentafein (1908). It constitutes a type specimen.
23 somites, Hertwig embryo “Wolff II,” Anatomisches-biologisches Institut, Berlin. Normal, well-preserved specimen, having three pharyngeal arches and no trace of rostral neuropore. Described by Keibel and Elze (1908). Specimen has been reconstructed.
23 somites, Carnegie No. 8964 (University of Chicago No. H 984). Described by Wen (1928) with particular reference to the nervous system.
24 somites, Johnson, Harvard Collection, Boston. Complete description, based on many models and histological study, published in monographic form by Johnson (1917). Rostral and caudal neuropores closed. Otic vesicle retains a narrow opening to surface. Three pharyngeal arches are present. Hepatic trabeculae invading framework of liver. Umbilical vesicle is compressed rostrocaudally, i.e., early umbilical stalk. Probably originally 25 somites (Arey, 1938).
24 somites, Homo Nürnberger, Anatomisches Institut, Universität Köln. Described in detail and excellently illustrated by Rosenbauer (1955), with particular reference to the cardiovascular system.
25 somites, West embryo, University College, Cardiff. A 3-mm embryo much like the Johnson specimen and a good representative of the middle period of this stage (West, 1937). By means of profile and wax reconstructions the main organ systems are outlined, including an excellent study of the nephric system. The success attained by West in the orientation of his sections and the consequent accuracy in profile outlines is explained by the stained margins of the squarely trimmed paraffin block, which served as guides.
25 somites, Carnegie No. 6097. A graphic reconstruction was published by Müller and O'Rahilly (1980a).
26 somites, His embryo M, Basel, H. h. 1. One of the group of embryos carefully studied by His for surface anatomy, and then cut in serial sections for microscopical examination, setting a new standard in human embryology (His, 1880–1885). From the development of the liver, lungs, heart, brain, and otocysts, and the absence of upper limb buds, it is estimated that it belongs in the 26-somite group.
28 somites, His embryo Lr., Leipzig No. 67. Although used to good purpose by His, this specimen is probably not entirely normal (His, 1880–1885). The estimate of 28 somites is based on the narrowed umbilical stalk and the beginning upper limb buds.
28 somites, Hammar embryo (Nystroem), Anatomisches Institut, Uppsala. Described by Hammar in Keibel and Elze (1908). Digestive system described by Forssner (1907). The number of somites given above is estimated on the basis of three pharyngeal arches, full convex back, small opening in otic vesicles to surface, hepatic trabeculae, and appearance of section through cardiac region. Central nervous system shows folding of wall which characterizes imperfect preservation. External form appears normal.
28 somites, von Spee collection, Kiel. Sketches published in Döderlein's Handbuch (1915). Reported to have 31 somites, but absence of upper limb buds and the fact that the otic vesicles are still open to the surface makes it probable Page 139 that an estimate of 28 somites is more nearly correct.
28–29 somites, Carnegie No. 148. Described by Gage (1905). Perhaps 29–30 somites (Arey, 1938).
29 somites, 20–21-day Coste embryo. Specimen not sectioned, but the exquisite drawings contained in Coste's atlas, Développement des corps organisés, Paris (1849), reveal many details of the surface form of this well-preserved embryo. In form it resembles closely Carnegie No. 1062, 29 somites, including rounded back curve, trace of upper limb buds, compressed gut-umbilical vesicle junction, size of hepatic area, form of cardiac tube, presence of otic pore, and outlines of head. Four pharyngeal arches are shown, but the fourth may have been an exaggeration of the depression lying caudal to the third bar. Also the somitic count seems to exceed the 29 estimated, but this may be caused by overemphasis on partial divisions of the terminal somitic ridge. In size its greatest length is about 4 mm. If it were straightened out as much as No. 1062, it would probably be close to 4.5 mm, like the latter.
29 somites, Janošík, Royal Bohemian University, Prague. Somitic count estimated on the following characteristics: three pharyngeal arches, closure of rostral and caudal neuropores, detachment of otocyst from surface, definite lung bud, elongated hepatic diverticulum with gut epithelium proliferating into adjacent tissue, narrowed umbilical stalk, and well-developed mesonephric duct and tubules. The main features of the vascular system are clearly shown. There were two embryos in this case, one of which was definitely stunted. The above description refers to the normal embryo Janošík, 1887).
29 somites, Waterston, University of St. Andrews, Fife. Specimen reported as having 27 paired somites (Waterston, 1914). In several characteristics it appears to be transitional between stages 12 and 13. Probably more than 27 somites, perhaps 28 (Arey, 1938) or 29. Among its advanced structures are prominent lung buds, large primordium of liver with extensive invasion by gut epithelium, narrow umbilical stalk, elongated median thyroid, and advanced ear vesicles. The upper limb buds were not prominent on the surface but stand out clearly in the sections. The blood vessels are everywhere greatly distended with blood cells, which is probably a peculiarity of this particular specimen.
The somitic count for the following embryos is not available.
Harvard No. 714, 4-mm embryo. Described in detail by Bremer (1906). Probably belongs to stage 12 rather than stage 11 or stage 13. It shows some unusual features, such as arrested or delayed closure of the rostral neuropore.
No. 102 and No. 126, Department of Anatomy, Tohoku University, Sendai. These two embryos were assigned to stage 12 but the somitic count is not given. Distribution of alkaline phosphatase was studied by Mori (1965).
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
