Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 6
Page 39Approximately 0.2 mm in size
Approximately 13 postovulatory days
Characteristic features: chorionic villi and secondary umbilical vesicle in 6a; primitive streak in 6b
The appearance of recognizable chorionic villi is used as the criterion for stage 6. The villi begin to branch almost immediately.
The space bounded by the internal surface of the chorion begins to expand greatly toward the end of stage 5 and during stage 6 (fig. 6-1). The secondary umbilical vesicle develops.
Axial features are not evident, or at least have not been described, in all embryos of stage 6. Moreover, in some instances, their presence is in dispute. It is possible, however, if the fixation and plane of section were always suitable, the series complete and free from distortion, and an adequate search made, that axial features would be found. Thus, in the well-known Peters specimen, which is frequently considered not yet to show axial features, the possible presence of an allantois was raised originally (Peters, 1899); apparently Grosser believed for a time that a primitive streak was present.
Hence it is convenient to distinguish (a) those embryos in which little or no axial differentiation has occurred or been noted, from (b) those embryos in which axial features, particularly a primitive streak, have definitely been recorded (fig. 6-10). This distinction corresponds more or less to Mazanec’s (1959) groups V and VI, respectively. In the latter, according to Mazanec, the chorionic villi have already begun to branch.
SIZE AND AGE
The maximum diameter of the chorion varies from 1 to 4.5 mm, that of its enclosed cavity from 0.6 to 4.5 mm. The embryonic disc varies from 0.15 to 0.5 mm in maximum diameter; the age is believed to be about 13 days.
It is of interest to note that those specimens (referred to here as 6a) in which definite axial features have not been described are characterized by a slightly smaller chorion (1-3 mm, as compared with 2-4.5 mm in the remainder, 6b), contained cavity (0.6-2.2 mm, as compared with 1.3-4.5 mm), and embryonic disc (0.15-0.22 mm, as compared with 0.15-0.5 mm).
HISTOLOGICAL FEATURES
A chart indicating the derivation of various tissues and structures is presented as figure 6-2.
Decidua. The decidua (fig. 6-8) varies considerably in thickness at the implantation site but is generally between 3 and 12 mm. A decidual reaction is present, a variable amount of edema and leucocytic infiltration is found, and actively secreting glands and prominent blood vessels are noticeable. Compact, spongy, and basal strata are distinguishable from superficial to deep (Krafka, 1941). At about this time the well-known subdivision of the decidua into three topographical components may be employed: (1) the decidua basalis is that situated at the deepest (embryonic) pole of the conceptus, (2) the decidua capsularis is reflected over the rest of the chorionic sac, and (3) the decidua parietalis lines the uterine cavity except at the site of implantation.
Chorion. The increasing structural complexity of the trophoblast from superficial to lateral and basal aspects has been attributed by Ramsey (1938) to the more advantageous nutritive conditions prevailing at the latter sites. The coalescence of the lacunae forms the intervillous space, which, placed as an offshoot on the uterine circulation, may be regarded as “a variety of arteriovenous aneurysm” (Hertig, 1968). The syncytiotrophoblast is more active enzymatically than the cytotrophoblast, and is believed, among other functions, to be responsible for the secretion of chorionic gonadotropin.
The cytotrophoblastic clumps and mesoblastic crests of stage 5 have now progressed to form processes that are commonly known as chorionic villi (fig. 6-3). Several Page 40 authors have pointed out that “the initial villi do not arise as free and separate outgrowths from the chorionic plate” into the lacunar spaces (Boyd and Hamilton, 1970). Trophoblastic trabeculae, which initially are syncytial in character, come to possess a central process of cytotrophoblast and are generally termed primary villi. None of the trabeculae, however, possesses a free distal end, because these “primary villous stems” do not arise as individual and separate sprouts from the chorion (Hamilton and Boyd, 1960) but rather by invagination of syncytiotrophoblast (fig. 6-3).

Fig. 6-1. Graph to show the progression in size from stage 1 to stage 8. Based on the measurements of 28 specimens. The interrupted lines indicate hypothetical values for stage 4. An enormous expansion of the chorionic cavity begins to take place toward the end of stage 5.
The mesoblastic crests form the cores of the villi, and the cytotrophoblastic clumps form caps from which cytotrophoblastic columns proceed externally. These columns, indications of which have been seen also at stage 5c, make contact with the stroma and form a border zone (penetration zone, Greenhill, 1927), the fetal-maternal junction, characterized by pleomorphic fetal cells (Krafka, 1941) and necrotic maternal cells. The columns also make contact with each other peripherally to form the cytotrophoblastic shell (fig. 6-3).
The cytotrophoblastic shell (of Siegenbeek van Heukelom) is the specialized, peripheral part of the cellular trophoblast in contact with maternal tissue. As the shell forms, syncytiotrophoblast is left both internally, where it lines the intervillous space, and externally, where it forms masses that blend with the decidua. The development and arrangement of the shell have been discussed by Boyd and Hamilton (1970). Defective development of the trophoblast, especially of the shell, results later in villous deficiencies (Grosser, 1926). Moreover, it is important to appreciate that, even in instances where the embryonic disc either fails to form or remains rudimentary, a large chorion with luxuriant villi may still be found.
Because the number of mitotic figures in the cytotrophoblastic covering of a villus is approximately equal to that in the mesoblastic core, Krafka (1941) suggested “that the mesoblast, once established, proliferates at the same rate as the Langhans layer, and hence furnishes its own growth zone.” The layer featured by Theodor Langhans (in 1870 and subsequently) is the villous cytotrophoblast, “which constitutes the cellular (as opposed to syncytial) investment of the villi” (Boyd and Hamilton, 1970).
Page 41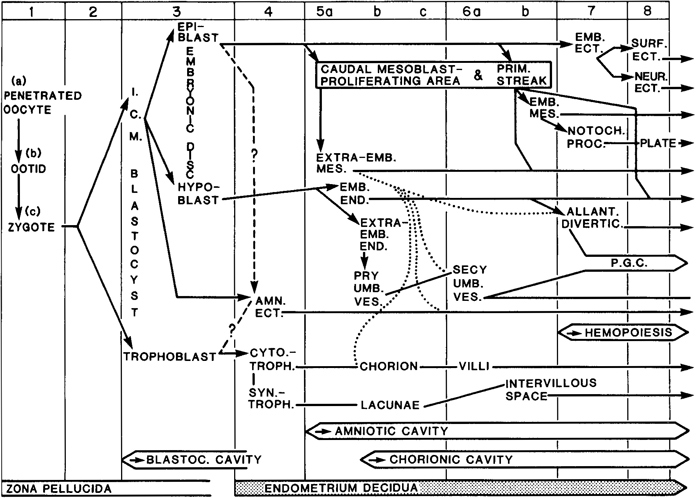
Fig. 6-2. Chart to show the probable sequence of development of various tissues and structures in the human embryo during the first eight stages. Some features are uncertain and others controversial, so that a definitive scheme will have to await further investigations. The various contributions of the extra-embryonic mesoblast to the chorion, amnion, secondary umbilical vesicle, and allantoic diverticulum are shown by dotted lines. In the mouse, it is now maintained that the hypoblast makes no contribution to the intra-embryonic endoderm. In the rhesus monkey, it is believed that the original epiblast gives rise to the amniotic ectoderm and the epiblast proper.
Park (1957) found a low incidence of sex chromatin in the trophoblast and chorionic mesoblast of an embryo (No. 7801) of stage 6, and in the chorionic mesoblast and umbilical vesicle of a second specimen (No. 7762).
Extra-embryonic mesoblast. The chorionic mesoblast is well formed and extends into the villi even in stage 6a, as seen clearly in the Linzenmeier and Peters specimens.
From his acquaintance with Hill’s observations of primates and from his own studies of the Fetzer embryo, Florian (1933) believed in “the existence of an area in the most caudal part of the embryonic disc where the primary mesoderm is fused with the ectoderm.” The zone in question may be the site “where primary mesoderm originates (at least in part). This area is situated close behind the primordium of the cloacal membrane” (ibid.), and involves the disc epiblast and the adjoining amniotic ectoderm. This theory of the origin of the primary mesoblast from a localized proliferating area has already been discussed under stage 5a.
At stage 6a, angiogenesis (see Hertig, 1935) is occurring in the chorionic mesoblast (Nos. 6900 and 6734), blood vessels are found in the villi (No. 6900) and blood islands are seen on the umbilical vesicle (No. 6734). By the end of stage 6b, vascularization of the chorion is almost constant and blood vessels are Page 42 generally present in the villi (ibid.). Thus, the blood vascular system first arises in extra-embryonic areas. From his studies of chorionic angiogenesis, Hertig (1968) became convinced of “the independent in situ origin of angioblasts and mesoblasts from trophoblast.”
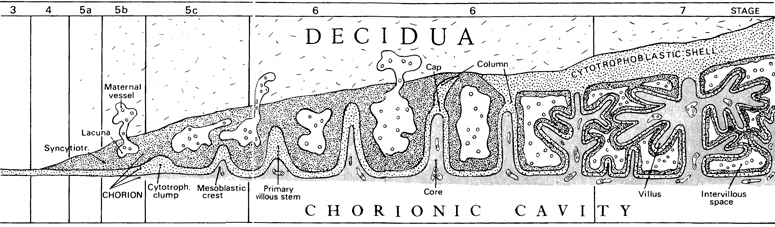
Fig. 6-3. Highly simplified scheme to show the preliminary events during stages 3-7 that lead to the development of the placenta. The cytotrophoblast (stage 3) gives origin to syncytiotrophoblast (stage 4), and lacunae appear in the latter (stage 5b). The appearance of a mesoblastic lining for the trophoblast results in a combination known as the chorion. Cytotrophoblastic clumps (stage 5c) acquire mesoblastic crests and become primary villous stems (stage 6). A cytotrophoblastic shell begins to form externally, and free villi project into the intervillous space (stage 7).
Amnion. The amnion is well formed. At its upturned margins, the epiblast of the embryonic disc changes abruptly to the squamous cells of the amnion (fig. 6- 9). Although the amnion appears largely as a single (ectodermal) layer, an external coat of mesoblast can also be detected and, in some places, it “runs along as a layer of mesothelium” (Heuser, Rock, and Hertig, 1945). The amniotic cavity may be either smaller or larger than the umbilical vesicle at stage 6 (fig. 6-4).

Fig. 6-4. Graph to show the maximum diameter of the amniotic cavity and of the umbilical vesicle from stage 5 to stage 8. Based on the measurements of 31 specimens. From stage 5c to stage 6, the primary is transformed into the secondary umbilical vesicle, although the mode of the transformation is not entirely clear. The secondary is at first smaller than the primary but soon enlarges considerably.
In the Fetzer embryo, among other specimens, Florian (1930a) was able to confirm von Möllendorff's finding in Op of enlargement of the amniotic cavity by epithelial degeneration. An active extension of the amniotic cavity toward the tissue of the connecting stalk took place in a dorsal and caudal direction from the embryonic disc.
The vault of the amniotic cavity may give rise to a diverticulum known as the amniotic duct. The appearances vary from a localized amniotic thickening (in No. 7801) to a pointed process directed toward the Page 43 trophoblast (in No. 7634, Krafka, 1941, fig. 2, and in No. 7762, Wilson, 1945, fig. 8). The amniotic duct is generally regarded as an inconstant and transitory developmental variation.
Embryonic disc. Terms such as “embryonic disc,” “embryonic shield,” Keimscheibe, Embryonalschild, or “blastodisc” are used in measuring embryos from the rostral amniotic reflexion to, frequently, the caudal end of the primitive streak (Odgers, 1941). Although certain authors (e.g., Grosser, 1931a) therefore do not include the cloacal membrane, others (e.g., Florian and Hill, 1935) do include it. When the connecting stalk and allantoic diverticulum are also included, terms such as “embryonic rudiment,” Embryonalanlage, Keimanlage, or Keimling are employed. Many writers do not make clear their points of reference, nor do they always specify whether a measurement has been taken in a straight line (caliper length) or along the curvature of the disc (contour length). In the present work, the “embryonic disc” will be taken to include, where possible, the cloacal membrane, and, except where otherwise specified, the length will generally refer to that measured in a straight line.
As seen from the dorsal aspect, the embryonic disc generally appears elongated, and the long axis of the disc usually (in ten out of twelve specimens of stage 6b) coincides with that of the primitive streak rather than lying at a right angle to it.
Although the dorsal surface of the disc may present some localized convexities and concavities, it is, as a whole, fairly flat. No. 7801, a particularly excellent specimen, is slightly convex (fig. 6-9) but the marked curvatures illustrated in some embryos (such as T.F.) may be assumed to be artifactual.
A detailed study of the “cytodesmata” in early human embryos was undertaken by Studnicka (1929). In two specimens (Bi I and T.F.) of stage 6, cytodesmata were described between the germ layers (“interdermal cy todesmata”) and between the mesothelial and the adjacent germ layer in both the amnion and the wall of the umbilical vesicle (Studnicka and Florian, 1928).
With the appearance of the primitive streak during stage 6, a process is begun whereby certain cells of the epiblast enter the streak, and the remaining cells Page 44 on the dorsal aspect of the embryo will become the embryonic ectoderm. The epiblast is continuous with the amniotic ectoderm at the margin of the embryonic disc. The basement membrane (membrana prima of Hensen) of the epiblast is clearly visible in a number of embryos of stage 6, such as Harvard No. 55 (where it has been demonstrated histochemically by Hertig et al., 1958) Peters, E.B., Bi I, etc.
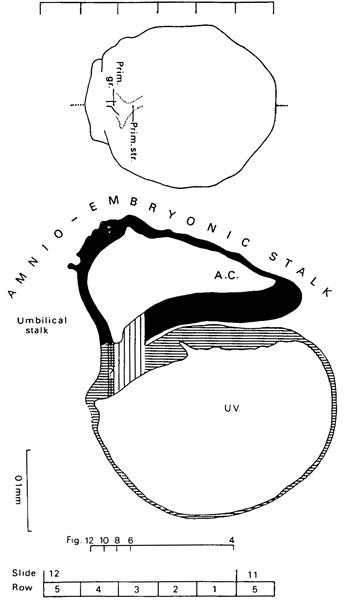
Fig. 6-5. Dorsal view and median reconstruction of No. 7801, stage 6, in alignment. The dorsal view, which is based on a graphic reconstruction by the present writers, shows the location of the primitive streak and primitive groove. The median reconstruction is based on a drawing by James F. Didusch (Heuser, Rock, and Hertig, 1945, Plate 3). The primitive streak is indicated by vertical lines, and the cross-hatched area (containing a question mark) suggests the site of the future cloacal membrane. The system of shading is further clarified in figure 7-1. The connecting stalk comprises amnio-embryonic and umbilical stalks. The figure references are to the sections reproduced by Heuser, Rock, and Hertig (1945, Plates 1 and 2).
The embryonic mesoblast will be discussed under the primitive streak.
The endoderm shows a marked concentration of glycogen, and some of its cells may be primordial germ cells (Hertig et al., 1958). Rostral to the primitive streak, the endoderm consists of large vesicular cells (Heuser, Rock, and Hertig, 1945). It should be kept in mind that, in the chick embryo, it has been shown that the rostral part of the primitive streak (including the node) always contains a large population of presumptive endoblastic cells.
Primitive streak. The primitive streak is a proliferation of cells lying in the median plane in the caudal region of the embryonic disc (figs. 6-5 and 6-10). The streak on its first appearance and in narrow usage, is “the thick caudal end of the germ disk’ (Heuser and Streeter, 1941). In a much broader and more functional sense, however, “the essential features of the primitive streak are the pluripotential nature of the cells that compose it and the continued segregation of more specialized cells which migrate or delaminate from the less specialized remainder” (ibid.).
The primitive streak enables cells from the outer layer of the embryo to pass inside and become mesoderm and endoderm (Bellairs, 1986). The process by which cells leave the epiblast, become part of the primitive streak, and then migrate away from the streak is termed ingression. It seems to depend on loss of the basal lamina beneath the streak.
On the basis of radio-autographic studies of grafts in chick embryos, the primitive streak is believed to be not a blastema but rather an entrance in which occur movement of epiblast toward the streak, invagination at the streak, and subsequent migration to both homolateral and heterolateral mesoderm. At primitive streak stages of the chick embryo, zones have been established for future ectoderm, mesoderm, endoderm, and notochord. It is likely that the mammalian pattern is basically similar. In the rabbit, the formation Page 45 of the embryonic disc and primitive streak “is primarily achieved by migrations of cells that are being rapidly proliferated over the entire surface of the embryonic area. Cell death occurs but is an insignificant factor in this phase of embryonic growth” (Daniel and Olson, 1966).
In the human, the primitive streak appears first during stage 6. The possibility of a streak in some embryos (e.g., Peters) here classified as 6a has been raised. Conversely a streak in at least one 6b embryo (No. 8819) has been denied (Krafka, 1941).
Brewer’s (1938) criteria for the presence of a primitive streak are (1) active proliferation of the cells (shown by a large number of cells in mitosis), (2) the loss of the basement membrane separating the epiblast and endoderm, (3) migration of the epiblastic cells, and (4) intermingling of the cells of the epiblast and endoderm of the disc.
The shape of the early streak is not entirely clear. Brewer (1938) described it as a crescentic formation at the caudal margin of the disc (similar to that seen in the pig), but, as already mentioned, the presence of a streak at all in his specimen (No. 8819) was denied by Krafka (1941).
In other young 6b embryos (Op, Fetzer, and Wo) the streak possesses the form of a node, and indeed initially “seems to correspond in its position with Hensen’s knot” (Florian, 1930a). Thus, in the Fetzer specimen (Fetzer and Florian, 1930) the streak appeared almost circular in dorsal view, was situated not far from the middle of the embryonic disc, and did not reach the cloacal membrane. The question naturally arises whether the primordium is not in fact the primitive node rather than the primitive streak in these early specimens. This idea is supported by Dr. J. Jirásek (personal communication, 1970), who believes that the fusion of the epiblast with the endoderm found in this region indicates that the node rather than the streak is involved. According to this interpretation, the primitive node appears during stage 6 and the streak would not appear until the following stage. In the chick embryo, although the streak is said to appear before the node, there is reason to believe that the rostral end of the very young primitive streak already contains the material of the future primitive node.
When the primitive streak attains a rostrocaudal measurement of 0.1 mm or more, as in Beneke, Am. 10, Bi I, and T.F., its elongation fully justifies the name “streak.” By the end of stage 6, both a node and a streak (separated by a “neck’) have been recorded in one (somewhat abnormal) embryo (HEB-18, Mazanec, 1960).
In the first specimens of stage 6b (Liverpool I and II, No. 7801, No. 8819, No. 7762, Op, Fetzer), the length of the primitive streak is less than one-quarter that of the embryonic disc. In Wo and Beneke it is less than one-third, and in Am. 10, Bi I, and T.F. it is less than one-half. Finally, in the transitional and somewhat abnormal HEB-18 specimen, the streak attains one-half the length of the embryonic disc.
The primitive groove appears probably during stage 6b. At any rate its presence has been claimed in some specimens (Liverpool II, Op, and T.F.) of that stage (fig. 6-10).
Although it may be possible, at least in some instances, to ascertain the rostrocaudal axis of the embryo at stages 5c and 6a, unequivocal manifestation awaits the initial appearance of the primitive streak during stage 6b (O’Rahilly, 1970). With the establishment of bilateral symmetry, the embryonic disc, in addition to its dorsal and ventral surfaces, now has rostral and caudal ends and right and left sides. The median plane may be defined,1 and the terms “medial” and “lateral” are applicable. Moreover, it is proper to speak of coronal (or frontal), sagittal, and transverse planes. The last-named, in anticipation of the erect posture, may be termed horizontal. Certain other terms, however, such as “anterior,” “posterior,” “superior,” and “inferior,” should be avoided at this period because of their special meaning in adult human anatomy.
1The terms “median sagittal” and “para-sagittal” are redundant. The median plane is sagittal by definition and anything parallel with a sagittal plane is still sagittal. This usage has been accepted in all the recognized anatomical terminologies.
Embryonic mesoblast. At first, the embryonic mesoblast is scarcely recognizable as such (Nos. 8819 and 7762) or is quite scanty in amount (No. 7801). Although the main bulk of the embryonic mesoblast is believed to come by way of the primitive streak, other sources are not excluded. Lateral to the streak, for example, it is possible that some epiblastic cells bypass the streak and migrate locally into the mesoblast (Heuser, Rock, and Hertig, 1945). Page 46 In addition, the possibility of contributions from the gut endoderm has been raised in the case of the macaque (Heuser and Streeter, 1941). Finally, the degree of incorporation of some of the primary mesoblast into definitive body mesoderm is unknown. Conversely, in the Beneke specimen, Florian (1933) “could trace the secondary mesoderm behind the caudal end of the primitive streak around the cloacal membrane into the connecting stalk.” (The very closely arranged cells of the secondary mesoblast were distinguishable from the looser cells of the primary tissue.) Hence, the convenient distinction between primary and secondary mesoblast should not be interpreted too rigidly. A need exists for further detailed studies of the distribution and spread of mesoblast during these early stages.
In the chick embryo, at the stage of the definitive streak, it has been established that each topographical kind of mesoblast has a definite place along the rostrocaudal axis of the primitive streak, precise enough to be demonstrated experimentally.
Prechordal plate. The earliest human embryo in which a definite prechordal plate has been recorded seems to be Beneke at stage 6b. In that specimen, “in front of, and below the cranial extremity of the primitive streak... the endoderm is distinctly thickened and proliferative” in “a horseshoe shaped area”; that area “must be regarded as a mesoderm producing zone” (Hill and Florian, 1963). Study of later specimens, such as Manchester 1285 and Dobbin, led Hill and Florian to “regard the thickened area in question as prechordal plate.” Dr. W. P. Luckett has called the writer’s attention to several stage 5c specimens (Nos. 7950,8558, and 8330) in which the endoderm appears to be thickened at one end of the embryo, as shown in the photomicrographs published by Hertig, Rock, and Adams (1956, figs. 35, 37, and 38).
It is of interest to note that, in Tarsius, the prechordal plate, which later adopts the form of an annular zone, is at first represented by a continuous sheet of thickened endoderm underlying most of the embryonic epiblast (Hill and Florian, 1963).
Umbilical vesicle (fig. 6-9). The umbilical vesicle functions “as a specialized nutritional membrane” (Streeter, 1937) and, in addition, serves as the site of origin of primordial germ cells as well as an important temporary locus of hematopoietic activity (Hoyes, 1969).
From stage 5c to stage 6, the primary is transformed into the secondary umbilical vesicle (fig. 6-4), although the mode of the transformation has long been disputed. (See Stieve, 1931; Heuser and Streeter, 1941; Strauss, 1945; Hamilton and Boyd, 1950; Starck, 1956.)
According to Hertig (1968), the primary vesicle “blows up” or “pops,” and the torn edges that remain attached to the endoderm coalesce to form the secondary vesicle. The secondary sac “soon takes on a second or inner layer of epithelial nature” which is, in Hertig’s view, derived from the wall of the sac itself. In other words, “endodermal” cells differentiate in situ from the wall of the umbilical vesicle (Streeter, 1937).
According to a number of other authors (such as Stieve, 1931), however, “it seems likely that endoderm from the edge of the embryonic disc proliferates and migrates round the interior” of the wall of the sac (Hamilton and Boyd, 1950), using that wall “as a guiding surface” (Mazanec, 1953).
A further possibility is the dehiscence of cells from the disc endoderm so that a new cavity is formed between the two endodermal layers or possibly between the disc endoderm and the dorsal part of the wall of the sac.
In any case, it appears likely that, at least in some embryos, the distal part of the primary umbilical vesicle becomes detached from the proximal part, thereby forming one or more isolated vesicles or cysts (see below). As a result of these processes, the secondary vesicle is at first smaller than the primary sac (fig. 6- 4). Thus the secondary vesicle may develop “as a result of the collapse of the abembryonic and lateral walls” of the primary sac, “with subsequent pinching-off and vesiculation of the distal collapsed portion” (Luckett, 1978). The part of the primary vesicle immediately under cover of the embryonic disc persists as the secondary umbilical vesicle.
It is maintained that the secondary vesicle is unilaminar in stage 6 and that adherent epithelial remnants of the primary sac merely give an impression that the secondary vesicle has already acquired an external mesodermal layer (Luckett, 1978).
In a number of embryos of stage 6 (such as No. 7634) and some subsequent stages, a diverticulum of the umbilical vesicle has been recorded. These outgrowths arise generally at the abembryonic pole of the main sac. They vary from slight evaginations to long Page 47 processes (0.45 mm in Liverpool II, for example), and are frequently associated with cysts. It has been suggested that the diverticula and cysts are remnants of the primary umbilical vesicle (Heuser, Rock, and Hertig, 1945).
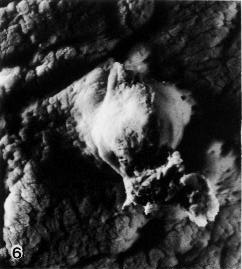
Fig. 6-6. Surface view of the implantation site of No. 7801. A small amount of maternal blood escaped and coagulated on one side of the elevated area.
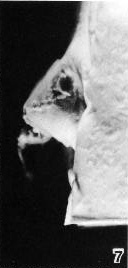
Fig. 6-7. Side view of the implantation site of No. 7801.

Fig. 6-8. General view of the tissues at and near the implantation site (No. 7801) to show the scab (Schlusscoagulum) of hemorrhagic exudate, actual hemorrhage underlying the scab, primary villous stems, blood-filled uterine glands, and early decidua. The embryo is evident, and, near the opposite wall of the chorion, a vesicle presumed to be a detached part of the umbilical vesicle can be identified. Section 12-1-1.
Cloacal membrane. The cloacal membrane appears during stage 6b. Although at least its site may be detected in the first specimens of that stage (such as No. 7801), the membrane is probably present in all specimens that possess a primitive streak 0.05 mm or more in length. The membrane is at first a cell cord that connects the epiblast with the endoderm (Florian, 1933) and is of variable length (about 0.015-0.025 mm). Later (stage 7) it increases in size and begins to assume the form of an actual membrane.
Allantoic diverticulum. The existence of an allantois in the human remained controversial until the end of the nineteenth century (Meyer, 1953). In early embryos, Page 48 the recognition of an allantoic primordium presents considerable difficulty. A recess of the umbilical vesicle, such as appears in embryo Op, should not be assumed to be necessarily the allantois. According to Florian (1930a), “the yolk-sac penetrates into the connecting stalk in the form of a narrow diverticulum which enlarges and eventually opens out again into the cavity of the yolk sac. This process may probably be repeated several times.” Hence some reservations must be made concerning the “allanto-enteric diverticulum” of Liverpool I. In No. 7801, all that is found is merely “a tiny recess in the wall of the yolk sac at the spot where the allantoic duct presumably originates” (Heuser, Rock, and Hertig, 1945). In Wo, a solid Allantoisanlage has been claimed. In Beneke, the diverticulum of the umbilical vesicle is not the allantois (Florian and Beneke, 1931). In Bi I, the appearance of the diverticulum has been attributed to “a ventral downbulging of the underlying wall of the yolk-sac” produced by the end node of the primitive streak (Florian, 1930a). The condition in T.F. may well be similar. In conclusion, it is difficult to find a convincing example of an allanto-enteric diverticulum at stage 6.
Connecting stalk. Florian (1930a) pointed out that the connecting stalk (as exemplified in embryo Op) comprises two portions (fig. 6-5): (1) the amnio-embryonic stalk, an attachment of the entire amnio-embryonic vesicle to the chorionic mesoderm, and (2) the umbilical stalk, by which the caudal end of the embryo is anchored to the chorion. The umbilical stalk, which is in all stages covered on its cranial surface by amniotic ectoderm, later becomes transformed into the umbilical cord.
The Peters embryo is situated in a thickening of the chorionic mesoderm but the connecting stalk is “not yet present” (Florian, 1930a). Although “a body stalk proper has not yet fully formed” in No. 7634 (Krafka, 1941), its primordium is present and comprises the amnio-embryonic and umbilical stalks of Florian. The condition is similar to that in Op, in which Florian has pointed out that the axis of the connecting stalk has already begun to form an acute angle (open caudally) with the embryonic plate. By the end of stage 6b, blood vessel primordia are present in the developing stalk and indicate the future umbilical vessels (Hertig, 1935). In the opinion of Hill and Florian (1963), the vessels of the connecting stalk in Tarsius “can arise directly as invaginations of the mesothelium” covering the stalk.
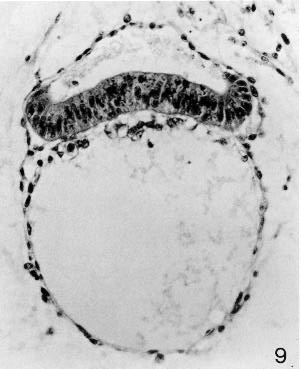
Fig. 6-9. The amniotic cavity, embryonic disc, and umbilical vesicle of No. 7801. The upturned margins of the epiblast change abruptly to the squamous cells of the amniotic ectoderm. The gut endoderm has a foamy appearance, whereas the cells lining the umbilicalvesicle are squamous. The umbilical vesicle appears to be acquiring an external coat. Section 12-1-1.
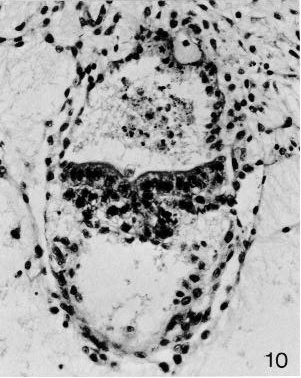
Fig. 6-10. The embryonic disc of No. 7801, in the region of the primitive streak. The connecting stalk, the amniotic cavity, and the umbilical vesicle are also visible. Section 12-3-6.
Page 49SPECIMENS OF STAGE 6a ALREADY DESCRIBED
Carnegie No. 8905, Merrill. Unbranched villi. Although an abnormal leucocytic reaction is present, this specimen “represents the best example in the author’s collection of formation of early primordial villi, active mesogenesis and angiogenesis, completion of the amnion and the transitional phase between the primary and definitive [secondary] yolk sac formation” (Hertig, 1968, who reproduced a photomicrograph as fig. 55). Presumed age, 12-13 days.
Carnegie No. 6800, Stöckel. Described by Linzenmeier (1914). Hysterectomy. Angiogenesis in chorion described by Hertig (1935). Photomicrographs reproduced by Hertig (1968), Figs. 56-58). Important as one of the youngest specimens having “true villi” (Hertig and Rock, 1941). Chorionic villi show “an occasional tendency to dichotomous branching” (Krafka, 1941). Indication of blood vessel formation in villi. Chorion, 2.75 x 1.05 x 0.9 mm. Chorionic cavity, 0.75 x 0.61 x 0.52 mm; capacity, 0.13 mm3 (Odgers, 1937). Embryonic disc, 0.21 x 0.105 mm (Krafka, 1941). Allantoic diverticulum doubtful. Presumed age, 13 days.
Carnegie No. 8672. Photomicrograph illustrated by Hertig, Rock, and Adams (1956, fig. 40). Chorion, 1.14 x 1.08 mm. Chorionic cavity, 0.8 x 0.79 mm. Embryonic disc, 0.203 x 0.07 mm. Presumed age, 13 days.
Harvard No. 55. Studied histochemically by Hertig et al. (1958). Hysterectomy. Chorion, 1.77 x 1.33 x 0.598 mm.Chorionic cavity, 0.73 x 0.68 x 0.221 mm. Embryonic disc,0.296 x 0.196 x 0.044 mm. Chorionic villi essentially solid,with earliest suggestion of mesoblastic core formation. “Apparentlywithout axial differentiation.” Possesses “a very recentlyformed definitive [secondary] yolk sac.” Possibleprimordial germ cells (“stuffed with glycogen”) within endodermnear edge of disc. Presumed age, 13 days. For histochemicaldetails, the original paper should be consulted.
Carnegie No. 8360. Photomicrographs illustrated by Hertig, Rock, and Adams (1956, figs. 42 and 46). Chorion, 1.466 x 1 mm. Chorionic cavity, 1 x 0.66 mm. Embryonic disc, 0.188 x 0.055 mm. Presumed age, 13 days.
Peters. Described in a monograph by Peters (1899). Autopsy. A famous embryo, for long the youngest known and the first to be described in detail. Photomicrographs have since been published (Rossenbeck, 1923, plate 42; Odgers, 1937, plate 2, fig. 2). The chorionic villi, some of which display a mesenchymal core, send cellular columns externally and these latter are beginning to form a cytotrophoblastic shell. Slight branching of villi (Krafka, 1941). Chorionic cavity contains magma réticulé of Velpeau (Mall, 1916). Blood islands on umbilical vesicle. Chorion, 1.5 x 2 mm. Chorionic cavity, 1.6 x 0.9 x 0.8 mm; capacity, 0.7 mm3 (Odgers, 1937). Embryonic disc, 0.18 x 0.24 mm (Krafka, 1941). The basement membrane (Hensen’s membrane prima) of the epiblast was noted by Graf Spee. Allantoic diverticulum and primitive streak uncertain. Presumed age, 13 days (Krafka,1941). A tabulation of normal human embryos compiled from the literature prior to 1900 and from Mall’s own collection was published by Mall (1900, pp. 38-46). The least advanced specimen was the Peters embryo, and included in the list were 92 embryos of 0.19-32 mm, as well as 17 fetuses of 33-210 mm.
E.B. (E. Béla v. Entz). Described by Faber (1940). Curettage. Incomplete. Primitive villi. Chorionic cavity, 0.935 x 0.697 mm. Embryonic disc, 0.231 mm. No primitive streak, node, or groove. Secondary umbilical vesicle. No allantoic diverticulum. Said to resemble the Peters specimen closely.
Carnegie No. 7634, Torpin. Described in detail by Krafka (1941), who provided also an extensive discussion of the decidua. Hysterectomy. Posterior wall of uterus. Chorionic villi with mesoblastic cores, 0.1-0.2 mm in length. Villi “generally single, but two or more may arise from a common base,” although no branching was recorded. Chorion (possessed 85 villi), 1.76 x 1.7 x 1.5 mm. Chorionic cavity, 1.3 x 1.1 x 1 mm. Embryonic disc, 0.216 x 0.21 mm. Amniotic duct. Neither primitive node nor primitive streak. Cloacal cord (rather than membrane) claimed, but doubted by Mazanec (1959). No allantoic diverticulum. Diverticulum of umbilical vesicle. Presumed age, 13 days. Dorsal and transverse projections published (Krafka, 1941, figs. 1 and 3, and plate 2).
VMA-I. Specimen of Knorre, summarized by Mazanec (1959). Chorion, 3.24 x 2.04mm. Chorionic cavity, 1.53 x 1.02 mm. Embryonic disc, 0.23 x 0.2 mm. Development thought to be between Torpin and Yale specimens.
Carnegie No. 6734, Yale. Described in detail by Ramsey (1938). Necropsy. Left lateral uterine wall. Chorion, 2.75 x 1.9 x 0.76 mm. Chorionic cavity, 1.3 x 1.1 x 1 mm. Some of the chorionic villi “show dichotomous division, but no more complicated branching has occurred.” Some angioblastic strands in villi. Embryonic disc (damaged and distorted), 0.15 mm. Allantoic diverticulum stated to be present but denied by Krafka (1941). Presumed age, 13-14 days. Drawings of model published (Ramsey, 1938, fig. 1).
Noback, Paff, and Poppiti (1968) described an autopsy specimen that possessed a chorion of 2.25 x 1.25 x 2 mm. Chorionic villi avascular. Embryonic disc, 0.22 x 0.2 mm. No primitive node, notochordal process, cloacal membrane, or allantoic diverticulum. Axial differentiation, however, was suggested by the possible primordia of the prechordal plate and the primitive streak. Hence this specimen may be regarded as transitional between 6a and 6b.
SPECIMENS OF STAGE 6b ALREADY DESCRIBED
Liverpool I. Described by Harrison and Jeffcoate (1953). Curettage. Chorionic villi “are only beginning to show evidence of branching” (ibid., Plate 1, fig. 1). Chorion, 1.86 x 1.47 mm. Chorionic cavity, 1.5 x 0.84 mm. Embryonic disc, 0.161 x 0.199 x 0.033 mm. Primitive streak, 0.021 mm. Allanto-enteric diverticulum claimed. Median projection published Page 50 (ibid., fig. 1; Mazanec, 1959, fig. 31).
Liverpool II. Described by Lewis and Harrison (1966), who, in view of “the dimensions, degree of differentiation and decidual appearances,” assigned the specimen to horizon VII. Hysterectomy. The chorionic villi are localized to the embryonic pole, and their mesoblastic cores contain “isolated vascular primordia formed by coalescence of angioblasts.” “The villi are branched; lacunae and intervillous spaces have formed.” Chorion, 2.72 x 2.35 x 1.54 mm. Embryonic disc, 0.264 x 0.22 mm. Primitive streak, 0.024 mm. Amniotic duct and long duct of umbilical vesicle. Resembles Teacher-Bryce II embryo. Median projection published (ibid., fig. 6).
Carnegie No. 7801 (figs. 6-5 to 6-10). Described in detail and illustrated by Heuser, Rock, and Hertig (1945). Hysterectomy Posterior wall of uterus. “The primitive villi are short and stubby; a few reach a length of about 0.25 mm.” Chorion, 2.6 x 1.9 x 1.4 mm. Chorionic cavity, 1.3 x 1.1 x 0.8 mm. Embryonic disc, 0.04 x 0.22 x 0.253 mm. Primitive streak, 0.04 mm. “Axial differentiation is just appearing.” “In this embryo the site of the future [cloacal] membrane seems indicated, but not the structure itself.” Probably no allantoic duct. Presumed age, 13-13½ days. Median projection published (ibid., plate 3).
Carnegie No. 8819, Edwards-Jones-Brewer. Described in detail by Brewer (1937, 1938). Hysterectomy. “There is no branching of a mesodermal villus.” Chorion, 3.6 x 3 x 1.9 mm. Chorionic cavity, 1.85 x 1.71 x 1.01 mm; capacity, 13.38 mm3. Embryonic disc, 0.209 x 0.177 mm; volume, 0.0814 mm3. Primitive streak, 0.04 mm, claimed, but its presence was denied by Krafka (1941). No allantoic diverticulum. Dorsal and median projections published (Brewer, 1938, plate 1, figs. 2 and 3; Mazanec, 1959, fig. 33). Some authors have attempted to identify a prechordal plate from the median projection.
Carnegie No. 7762, Rochester. Described by Wilson (1945). Curettage. Chorion, 2.3 x 2.2 x 2 mm. Larger villi have a mesoblastic core “and some show a tendency toward branching.” Moreover, “no evidence of actual blood vessels is seen in the villi, but in many of them groupings of angioblasts are observed.” Chorionic cavity, 1.75 x 1.3 x 1 mm. Embryonic disc, 0.313 x 0.22 mm. Primitive streak, 0.04 mm. No definite allantoic diverticulum. Amniotic duct. Median reconstruction published (ibid., plate 3; Mazanec, 1959, fig. 36).
Op (Opitz). Described by von Möllendorff (1921b). Hysterectomy. Chorionic villi show first branching in many places. Chorionic cavity, 1.5 x 1.15 x 1 mm. Embryonic disc, 0.19 mm. Primitive streak, 0.045 mm. Allantoic diverticulum denied by Florian (1930a). Disintegrating epithelial proliferation of amnion behind caudal end of embryonic plate (ibid.). Median reconstruction published (Mazanec, 1959, fig. 34).
Fetzer. Described by Fetzer (1910) and by Fetzer and Florian (1929, 1930). Curettage. Chorionic villi “show a beginning tendency to branch” (Streeter, 1920). Chorion, 2.2 x 1.8 mm. Chorionic cavity, 1.6 x 0.9 mm. Embryonic disc, 0.26 x 0.215 mm, Primitive streak (denied by Rossenbeck, 1923), 0.05 mm. Cloacal membrane (Florian, 1933) but no allantoic diverticulum. Area of mesoblastic proliferation from adjacent disc and amniotic ectoderm, “caudal” to cloacal membrane (Hill, 1932; Florian, 1933). Stated to lie between Wo and Bi I in development. Dorsal and median projections published (Fetzer and Florian, 1930, figs. 1a, 1b, 2 and 53; Florian, 1945, plate 4, fig. 40; Mazanec, 1959, fig. 37).
H.R. 1 (Hesketh Roberts). Described by Johnston (1940) who included Florian’s divergent interpretation of the specimen Hysterectomy. Chorion and endometrium described by Johnston (1941). According to Florian, the embryonic disc is 0.048 mm and the primitive streak is 0.06 mm in length. Primitive node, notochordal process, and prechordal plate all absent (but described as present by Johnston). Embryo abnormal in shape, the result of an abnormal growth process. Median projection published (Johnston, 1940, fig. 35).
Wo (Wolfring). Described by von Möllendorff (1925). Chorionic cavity, 2.52 x 2.16 x 2.06 mm. Embryonic disc, 0.25 x 0.22 mm. Primitive streak, 0.065 mm. Cloacal membrane rather than solid allantois (Florian, 1933). Median projection published (von Möllendorff, 1925, fig. 4; Florian, 1928a, fig. 40; Mazanec, 1959, fig. 38).
Beneke (Strahl-Beneke). Described originally by Strahl and Beneke in 1916 in a monograph and later by Florian and Beneke (1931). Chorionic cavity, 3.8 x 2.2 x 1.2 mm. Embryonic disc (narrow type), 0.375 mm (Florian, 1934a). Primitive streak (doubted by Rossenbeck, 1923, and denied by Fahrenholz, 1927, but acknowledged by Florian, 1928a), 0.1 mm. No notochordal process (Hill and Florian, 1931b). Prechordal plate, 0.066 mm. Dorsal and median projections published (Florian and Beneke, 1931, figs. 2 and 1; Florian, 1928a, fig. 42; Florian, 1945, plate 4, fig. 41; Mazanec, 1959, fig. 40).
Am. 10. Described by Krause (1952). Hysterectomy. Chorionic cavity, 3.6 x 2.5 x 2.5 mm. Embryonic disc (broad type), 0.32 x 0.3 x 0.06 mm. Primitive streak, 0.135 mm. No notochordal process (but see Mazanec, 1959) although a small lumen was suggested as a possible Anlage of “Lieberkühn’s canal,” Dorsal and median projections published (Krause, 1952, figs. 13 and 15; Mazanec, 1959, fig. 42). Could be stage 7.
Bi I (Bittman). Described by Florian (1927) and in 1928 in a Czech publication, (For general appearance, see Mazanec, 1959, figs. 95 and 112.) Chorionic cavity, 2.13 x 2.13 x 2.12 mm. Embryonic disc (broad type), 0.35 x 0.34 mm. Primitive streak, which appears as an “indifferent cellular knot” (Florian, 1928b, 0.135 mm). Possible primordial germ cell in ventral wall of umbilical vesicle (Politzer, 1933). Median projection published (Florian, 1928a, fig. 41; Florian, 1945, plate 5, fig. 42; Mazanec, 1959, fig. 41).
Lbg (Lönnberg). Described by Holmdahl (1939). Chorion, 16 x 15 mm. Embryonic disc, 0.285 x 0.236 x 0.032 mm. Primitive streak, 0.144 mm. No allantoic duct. T.F. Described by Florian (1927, 1928a). Autopsy. Chorionic Page 51 cavity, 4.578 x 3.078 x 1.76 mm. Embryonic disc, 0.468 x 0.397 x 0.485 mm. Primitive streak (Mazanec, 1959, fig. 100) 0.162 mm. No notochordal process. Median projection published (Florian, 1928a, figs. 27 and 43; Mazanec, 1959, fig. 43).
HEB-28. Described by Mazanec (1960). Abortion. Abnormal features, Chorionic cavity, 4.29 X 4 X 3.55 mm. Embryonic disc (broad type), 0.44 X 0.47 mm. Primitive streak, 0.187-0.22 mm, and node, 0.071 mm. No notochordal process. Dorsal and median projections published (ibid., figs. 1 and 2). Regarded as transitional between stages 6 and 7.
ADDITIONAL SPECIMENS
Precise measurements of the primitive streak have not been provided in accounts of the following embryos. The specimens are listed in order of year of publication.
Minot. Described by Lewis in Keibel and Mall (1912). Primitive streak present. Median projection published (ibid., vol. 2, fig. 229).
Schlagenhaufer and Verocay (1916) described an autopsy specimen that possessed an embryonic disc of 0.24 X 0.28 mm. Although a primitive streak was not found, the development of the specimen is such that it was probably present (Mazanec, 1959).
Teacher-Bryce II. Described by Bryce (1924) and M’Intyre (1926). Autopsy. Chorionic villi are “well developed but are still simple and little branched.” Chorion, 4.5 X 4 X 3.5 mm. Chorionic cavity, 2.8 X 2.6 X 2.25 mm. Embryonic disc, 0.2 X 0.1 X 0.15 mm. Primitive streak present (Mazanec, 1959). Long stalk from umbilical vesicle. Blood vessel primordia in connecting stalk.
H381. Described by Stump (1929). Chorionic villi branched. Chorion, 4.38 x 4.2 x 1.4 mm. Chorionic cavity, 3.48 X 3.44 X 0.81 mm. Embryonic disc, 0.58 X 0.3 mm. Primitive groove and streak or node believed to be present. Said to resemble Hugo and Debeyre specimens.
Andô Described by Hiramatsu (1936). Hysterectomy. Chorion, 4.2 X 3.25 X 1.9 mm. Chorionic cavity, 4.2 X 2.4 X 1 mm. Embryonic disc, 0.24 X 0.26 x 0.04 mm. Some villi branched. Stated to resemble the Peters specimen. Described as possessing no primitive streak but Mazanec (1959) detected a very early Anlage of the primitive streak in one of the illustrations. Presumed age, 14-15 days. A median interpretation has been published (Mazanec, 1959, fig. 35).
Carnegie No. 6026, Lockyer. A pathological specimen described by Ramsey (1937). Necropsy. Branching villi. Chorionic cavity, 2.12 X 1.48 x 1.6 mm. Embryonic disc degenerated. Primitive groove and embryonic mesoblast probably present. Formerly classified under horizon VIII.
Thomson. Described by Odgers (1937). Chorionic cavity, 2.1 x 1.51 x 0.7 mm; capacity, 1.55 mm3. Embryonic disc (which shows “a good deal of disorganization”), 0.26 X 0.31 (?) X 0.16 (?) mm. Compared by author to various embryos of stage 6.
Fife-Richter. Described briefly in an abstract by Richter (1952). Hysterectomy. Branching villi. Chorion, 3.44 mm. Chorionic cavity, 2.24 mm. Embryonic disc, 0.29 X 0.4 mm. “A poorly defined primitive streak with groove is present.”
Kistner (1953) had only one slide through the embryonic disc. Curettage. Primitive streak thought to be present. Presumed age, 13 days.
Jahnke and Stegner (1964) described a specimen that possessed a chorionic cavity of 2.5 x 2.3 x 1.5 mm. Embryonic disc, 0.31 X 0.29 mm. Primitive streak not precisely ascertainable but thought to be present. Presumed age, 15-16 days. Median projection published (ibid., fig. 2).
Hamilton, Boyd, and Misch (1967) described twins. Hysterectomy. Chorion 2.37 X 2 x 1.4 mm. Early villi. Embryonic disc with primitive node. Twin represented by (1) a vesicle interpreted as “a poorly developed embryonic disc and amnion,” and (2) a detached umbilical vesicle. Monozygotic twinning here probably caused by unequal division of inner cell mass of blastocyst.
Carnegie No. 8290. Abnormal specimen illustrated by Hertig (1968, fig. 129). Polypoid implantation site, deficient polar trophoblast, and buckled embryonic disc. Presumed age, 13 days.
Carnegie No. 7800. Abnormal specimen illustrated by Hertig (1968, fig. 126). Trophoblastic hypoplasia with virtual absence of chorionic villi. Presumed age, 13 days. Thought to belong to either stage 6 or stage 7.
Liverpool III. Described by Rewell and Harrison (1976). Some chorionic villi branched. Embryonic disc, 0.238 mm. Primitive streak.
Several other unsatisfactory specimens (in poor condition or inadequately described or both) have been published but will not be referred to here. These include Bayer (Keibel, 1890) and von Herff (von Spee, 1896), and the specimens of Giacomini (1898), van Heukelom (1898), Jung (1908) Herzog (1909), Heine and Hofbauer (1911) Johnstone (1914), Greenhill (1927), and Thomas and van Campenhout (1953). These probably belong to stage 6, and further examples may be found in Mazanec (1959). Moreover, frankly pathological specimens have also been recorded, e.g., by Harrison, Jones, and Jones (1966).
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
