Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 8
Page 65Approximately 1–1.5 mm in greatest length
Approximately 18 +/- 1 postovulatory days
Characteristic features: presomitic; primitive pit; notochordal canal
Stage 8 is characterized by the appearance of one or more of the following features: the primitive pit, the notochordal canal, and the neurenteric canal. The embryo is presomitic, i.e., somites are not yet visible.
In Streeter’s (1942) scheme, horizon VIII was characterized by “Hensen’s node, primitive groove.” Both these features, however, are present in embryos already assigned to horizon VII (e.g., No. 7802) and may even be found in at least some stage 6b specimens. It will be noticed, therefore, that the criteria employed here for stages 7 and 8 are not those of horizons VII and VIII, respectively; a similar statement will apply to stage 9 and horizon IX.
By this time, the third week of development, the uteroplacental circulation is well established, the decidua well formed, and the decidua capsularis healed over the conceptus (Hertig, 1968). Very much later, namely, at midterm, the decidua capsularis will fuse with the decidua parietalis, thereby obliterating the uterine cavity.
A fairly high incidence of sex chromatin has been recorded (Park, 1957) in the notochordal process and umbilical vesicle of two embryos of stage 8 (Nos. 7666 and 7701), and a lesser incidence in the amnion, trophoblast, and chorionic mesoblast, whereas none was found in two other specimens (Nos. 7949 and 8727).
Although doubts may be raised in regard to certain other specimens, the following definitely belong to stage 8 and may be taken as representative of that stage: Shaw, Kl.13, Dobbin, No. 5960, No. 7640, Peh. 1-Hochstetter, R.S., Western Reserve 1, Gläveke, and Strahl. Drawings of No. 5960 are provided in figures 8-3, 8-4, and 8-5.
A detailed investigation of this stage was undertaken by O'Rahilly and Müller (1981), who provided graphic reconstructions.
SIZE AND AGE
The maximum diameter of the chorion varies from 9 to 15 mm, that of the chorionic cavity from 3 to 10 mm (fig. 8-1). The embryonic disc is approximately 0.5–2 mm in length, and the age is believed to be about 18 days.
EXTERNAL FORM
The embryonic area is pyriform, being broader rostrally and tapering caudally. It may be possible to see the primitive node and the notochordal process from either dorsal (fig. 8-6) or ventral (fig. 8-7) view. The dorsal surface of the disc is slightly convex.
With the exception of those specimens that are either broad (No. 8820, Schö) or particularly elongated (Dobbin, Western Reserve 1), the dorsal surface of the embryonic disc appears ovoid (Wa 17) or pyriform (No. 5960). The interesting idea that two different types of young embryos occur was taken up and elaborated by Florian (1934a), who compared a “dwarf form” (Zwergform: Beneke, Bi 24, Wa 17, Western Reserve 1) with a “giant form” (Riesenfom: Bi I, Hugo, Schö, Peh. 1-Hochstetter) at stages 6–8. O’Rahilly (1973) calculated an index (width x 100 divided by length) for 55 specimens from stage 5c to stage 11. The result showed that eleven specimens had an index of more than 100 (broad specimens), one was at 100 (circular specimen), and 43 were below 100 (narrow specimens). Moreover, a gradual, relative elongation took place from stage 6 to stage 11. Specimens of stage 5c, which did not show definitive axial features, did not fit into the pattern. It may be concluded that Florian’s study has served to emphasize that, from stages 6 to 8, wide Page 66 variations in shape occur and embryos may be squat or, more commonly, elongated. As in the case of bodily habitus in the adult, however, a range is found, and it would be an oversimplification to attempt to force all examples into Laurel and Hardy types. Both the embryo and the adult are pleomorphic rather than dimorphic.

Fig. 8-1. The range of various measurements at stages 5 to 8. Based on 86 specimens (3 at stage 5a, 3 at 5b, 9 at 5c, 10 at 6a, 17 at 6b, 22 at 7, and 22 at stage 8). The two left-hand graphs are at a scale ten times greater than the others. It can be seen that the chorion attains a maximum diameter of 10 mm and the embryonic disc a length of 1 mm during stage 8.
HISTOLOGICAL FEATURES
Decidua. The conceptus is embedded in the stratum compactum, which is sharply defined from the stratum spongiosum (No. 8820, Jones and Brewer, 1941, plate 4, fig. 10).
The first maternal vessels in communication with the lacunae appear to be venules or capillaries, so that the circulation in the lacunae is initially sluggish. It is said that “no direct arterial openings into the intervillous space are found in presomite embryos” (Hamilton and Boyd, 1960).
Amnion (figs. 8-8 and 8-12). The structure of the amnion resembles that seen in the previous stage. It is possible that “many of the cells of the amnion in the region of the peak undergo retrogressive changes and are sloughed into the amniotic cavity” (Jones and Brewer, 1941).
Embryonic disc (fig. 8-5). The ectodermal cells, mostly tall and columnar, rest upon a basement membrane except in the line of the axial formation. The transition to the flat cells of the amniotic ectoderm is sharp. Cells in mitosis are frequent. Small, deeply stained protein coagulatory granules are taken to be evidence of necrosis, which is said to be “noted in all normal young embryos” (Jones and Brewer, 1941). It may be mentioned Page 67 in passing that cell deaths in normal human ontogeny have been studied chiefly in embryos from 3 mm in length onward (Ilies, 1967).

Fig. 8-2. Simplified scheme of the probable modes of development of canalization in the primitive node and in the notochordal process. The notochordal canal is formed at stage 8 and extends from the primitive pit into the notochordal process (as in the Shaw embryo). The floor of the canal breaks down almost immediately (as in Kl. 13, Peh. 1-Hochstetter, and Western Reserve No. 1), and the neurenteric canal appears (as in Dobbin and R.S.). All the specimens shown here are human, with the exception of Loris No. 49, The neurenteric canal, which first appears during stage 8, may still be found in certain embryos of stages 9 (Da 1) and 10 (No. 3709), but not in others (No. 1878 and No. 5074).
Endoderm. The endoderm presents gentle elevations and depressions as seen from the ventral aspect, and its dorsal surface, “due to numerous extensions which lead toward the mesoderm, has the appearance of a range of mountains” (Heuser, 1932b).
By stage 5c the endoderm appears to be complete, i.e., it lines the umbilical vesicle and the ventral aspect of the embryonic disc. In stage 6b, when the primitive streak and notochordal process form, the endodermal lining appears incomplete. No endoderm is present adjacent to the primitive streak (as shown by Brewer, 1938, in the Edwards-Jones-Brewer embryo) and notochordal process. The same is true for stage 7 (as shown by Hill and Florian, 1931b, Florian and Hill, 1935, and Florian, 1945, in the Hugo, Bi 24, and Manchester 1285 embryos). The situation becomes more complex in stage 8, when the endoderm begins to grow caudorostrally in the area of the primitive streak and node (as shown by Hill and Florian, 1931b, and Heuser, 1932, in the Dobbin, No. 5960, Peh. 1-Hochstetter, and Western Reserve 1 embryos).
Page 68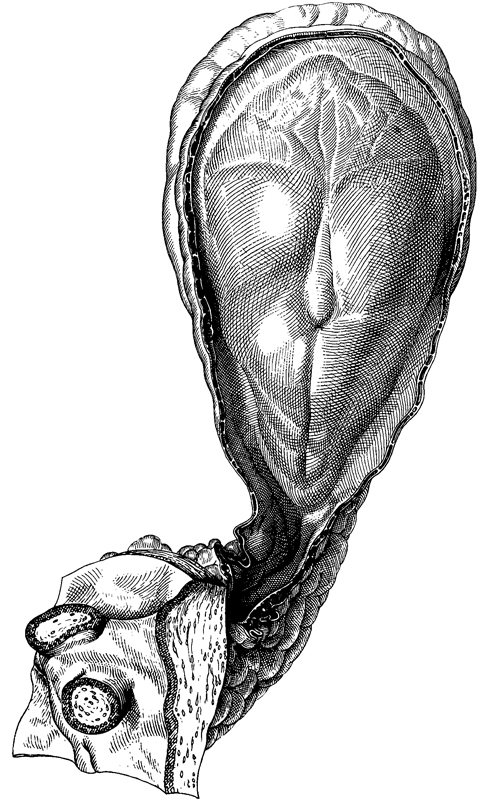
Fig. 8-3. Dorsal view of embryo No. 5960, drawn by James F. Didusch from a reconstruction. The amnion has been cut, and, on the dorsal surface of the embryonic disc, the neural groove, primitive node, and primitive groove can be identified in rostrocaudal sequence. A portion of the umbilical vesicle can be seen rostrally, and two chorionic villi appear in section in the lower left-hand corner.
Page 69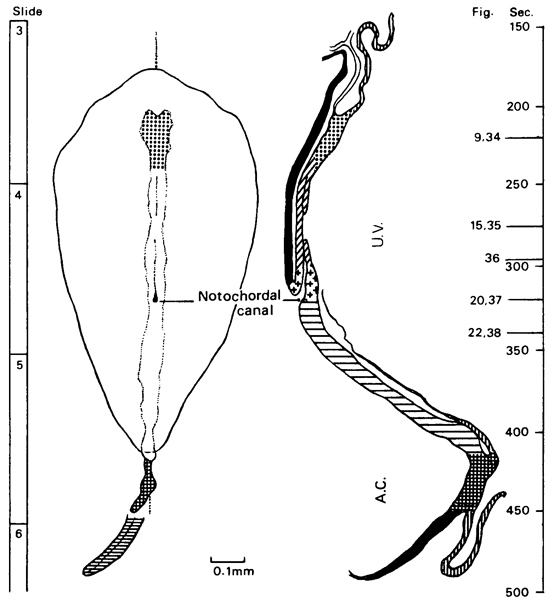
Fig. 8-4. Dorsal view and median reconstruction of No. 5960, stage 8, in alignment. The dorsal view, which is based on drawings by James F. Didusch (Heuser, 1932b, figs. 33 and 40), shows the prechordal plate, notochordal canal and primitive pit, cloacal membrane, and allanto-enteric diverticulum. The location of the prechordal plate is shown according to Heuser, and differs from the interpretation of Hill and Florian, who believed that the plate should be situated further rostrally. The system of shading is that shown in figure 7-1. The median reconstruction is based on a drawing by James F. Didusch (Heuser, 1932b, fig. 47). The notochordal canal can be seen to proceed from the primitive pit through the notochordal process, where the floor of the canal has disintegrated in its middle portion. Neither foregut nor hindgut is yet delineated. The allanto-enteric diverticulum can be seen caudally in close relation to the cloacal membrane. The figure references are to the sections reproduced by Heuser (1932b, plates 2 to 5).
A clear succession is established whereby the least developed embryos have either no endoderm or a more restricted extent of it ventral to the primitive streak and node, as shown by O’Rahilly and Müller (1981, fig. 4): D (No. 9251), C (No. 9286), K (No. 9009B), E (No. 9123), B (No. 9009A), A (No. 8725), H (No. 5960), F (No. 8671), and G (No. 7545). In the last three cases the endoderm reaches as far as the primitive node. The most advanced embryos with regard to endodermal spread are also more advanced in other respects. Page 70 (Nos. 5960 and 7545 already show a neural groove.) It may be that this endoderm is derived from the caudal part of the primitive streak (fig. 6-2).
The area ventral to the notochordal process/plate remains free of endoderm also during stages 9 and 10. Only in stage 11, at the time when and in those places where the definitive notochord will form, does the endoderm become complete in the medial part of the embryonic intestinal roof. The mitotic figures giving rise to new endodermal cells are clearly found in the endoderm adjacent to the transforming notochordal plate (Müller and O’Rahilly, 1986c, fig. 2).
Neural plate and groove. The general area of the neural plate, comparable to that shown experimentally in the chick embryo, can be assessed (O’Rahilly and Gardner, 1971) at stages 7 and 8. Indeed, areas for the probable location of the future epidermis, neural crest, alar plate, and basal plate can be conceptualized in accordance with the scheme used for the chick blastoderm.
In amphibian embryos it has been shown that the formation of the neural plate is “induced” by the “chordamesoderm,” Spemann’s organizer.
The neural groove (figs. 8-9 and 8-17) appears first in the more advanced specimens of stage 8: Nos. 5960, 7545, 7640, and 10157. These embryos possess a notochordal process longer than 0.4 mm. Another embryo (R.S.) is in very poor histological condition (Odgers, 1941), and further examples are either unconvincing (Triepel, 1916) or lack adequate mensural data (Cordier and Coujard, 1939). The neural groove has been recorded also in the M’Intyre and Gläveke embryos, which are very advanced in stage 8 and may even belong to stage 9.
The extent of the neural groove seems to be correlated not only with the length of the notochordal process but also with its shape. Four embryos that displayed a neural groove were also the only four out of eleven specimens in which the floor of the notochordal process was breaking down and a notochordal plate was present (O’Rahilly and Müller, 1981). Moreover, the neural groove was, in general, coextensive with the notochordal plate, reaching slightly more rostrally in two cases. Furthermore, the width of the neural groove corresponded approximately to that of the notochordal plate.
In summary, the first visible indication of the nervous system in the human embryo is the appearance of the neural groove during stage 8 (O’Rahilly and Müller, 1981). It appears before the heart or any of the other organs becomes visible.
Primitive node and streak. The primitive node has been recorded in most specimens. It varies from 0.03 to about 0.06 mm in length. The node may project above the surface, and it may (Nos. 5960 and 7640) be separated from the streak by a neck. In the intact embryo, the primitive node stands out as an opaque white spot (fig. 8-6). The node (fig. 8-2) may be merely indented by the primitive pit (Schö), possess some cavities (No. 8820), or be penetrated by a notochordal canal (Shaw). The cells of the node are large, possess more or less spherical nuclei, and tend to be arranged radially (Jones and Brewer, 1941). A plug (Dotterpropf) has been described immediately caudal to the dorsal opening of the notochordal canal (Rossenbeck, 1923).
The primitive streak (figs. 8-12 and 8-15) varies from 0.05 to 0.7 mm in length.
Embryonic mesoblast. Mesoblastic cells have by now spread beneath the entire surface of the ectoderm to reach the margin of the embryonic disc, where fusion with the extra-embryonic mesoblast occurs. The cellular density of the embryonic mesoblast is greatest near the primitive streak (Jones and Brewer, 1941, plate 5, fig. 11). Some mesoblastic cells are probably being contributed by the endoderm also (Heuser, 1932b). The extent of the mesoblast, however, is very variable (Grosser, 1934).
Somites. The initial appearance of somites is difficult to detect and is scarcely possible in transverse sections. Hence certain specimens that show very advanced features (e.g., a pericardial cavity) may well have possessed a pair of somites and may belong to stage 9 rather than to stage 8.
Coelom. Some isolated spaces are beginning to form in the mesoblast (in No. 5960) and, “from their position in the pericephalic region (figs. 6, 7, and 12) it is evident that they represent the pericardial cavities...” (Heuser, 1932b). Although such isolated spaces have been interpreted as the pericardial cavity, “no precardiac splanchnic cells could be identified in relation to such cysts,” so that a pericardial cavity cannot be identified with certainty at stage 8 (de Vries, 1981).
Page 71
Fig. 8-5. Five transverse sections through No. 5960, stage 8, to show the arrangement of the germ layers and of the various axial features of the embryo. The levels of the sections A to E are shown also on a dorsal view of the intact embryo (inset drawing). The photomicrographs on which these sections are based are reproduced here in figures 8-8 to 8-12.
Page 72
Fig. 8-6. Dorsal view of No. 5960, photographed in formalin. The primitive node appears as a conspicuous opaque knot from which the notochordal process extends rostrally. The umbilical vesicle is clearly visible in the upper third of the photograph, and the connecting stalk and adjacent chorion can be seen in the lower third.

Fig. 8-7. Ventral view of No. 5960, photographed in formalin. The notochordal plate can be detected. The umbilical vesicle is clearly visible in the upper third of the photograph, and the connecting stalk and adjacent chorion can be seen in the lower third.
When the spaces coalesce, the typically U-shaped pericardial coelom is formed. This has been plotted by M’Intyre (1926, text-fig. 7) but this advanced specimen may belong to stage 9. In another advanced embryo (Gläveke), which may also belong to stage 9, “a true typical endothelial anlage” of the heart, situated between the endoderm and the mesoblast, has been illustrated (Evans in Keibel and Mall, 1912, fig. 404). In summary, embryos that show a pericardial cavity or an indication of the heart belong probably to stage 9 rather than stage 8.
Notochordal process. The notochordal process, which varies from 0.01 to 0.8 mm in length, is fused with the endoderm and is in contact laterally with the mesoblast. Cytodesmata may be seen between the notochordal process and the overlying disc epiblast. The fully formed notochordal process, as seen in the Dobbin embryo, has been described as consisting of three portions (Hill and Florian, 1931b): (1) the rostral part, an undifferentiated cell mass, (2) the middle part, comprising a median, canalized process and two lateral mesoblastic bands, and (3) the caudal part, which lacks the lateral wings but possesses the notochordal canal.
Notochordal and neurenteric canals. The notochordal (or chordal) canal (of Lieberkühn) is initially indicated (fig. 8-2) merely by the primitive pit (in Schö) or by some cavities in the primitive node (in No. 8820). In its typical form, it extends from the primitive pit into the notochordal process (in Shaw), where the cells of the process are arranged around it in a radial manner (fig. 8-13). The intact canal is of very brief duration, however, and the floor of the canal, which becomes intercalated in the endoderm, begins to disintegrate at once in several places.
It should be stressed that the term “notochordal canal” is used for the canal in the notochordal process, that is, for the Chordafortsatzkanal or Kopffortsatzkanal (discussed by Springer, 1972), which is quite different from any cavity that may subsequently appear during the folding of the notochordal plate, an event that does not begin until during stage 10.
Already during stage 8 (fig. 8-2), in some areas (as many as seven in the Dobbin embryo) the floor of the notochordal canal may have disappeared (Kl.13, Peh. 1-Hochstetter, Western Reserve 1) so that the canal becomes replaced in part by a groove that opens ventrally into the umbilical vesicle. Rostrally, the canal reenters the tip of the notochordal process. The groove is situated in the notochordal plate, which is intercalated Page 73 into the endoderm and, by increasing in thickness and breadth, appears to be more than “merely the remains of the dorsal wall of the chorda canal” (Odgers, 1941). Furthermore, “as Bryce and others have insisted, the breadth [of the plate] suggests that it must yield something more than simply the notochordal rudiment, i.e. that it probably helps to form the entoderm of the digestive” system (ibid.).
“It is probable that the disappearance of the ventral wall of the chorda-canal is subject to great individual variation, and cannot be used by itself as an infallible guide in estimating the stage of development” (Hill and Florian, 1931b).
The notochordal canal takes a course that goes from oblique to horizontal. With increasing breakdown of the canal floor a vertical (perpendicular to the disc) passage appears (in Dobbin and R. S.) and is known as the neurenteric canal (Odgers, 1941). Both canals commence in common dorsally in the primitive pit, and the neurenteric canal may be regarded as the remains of the notochordal canal at the level of the primitive node (Van Beneden, 1899).
Prechordal plate (figs. 8-8 and 8-16). The prochordal plate is a localized area of endoderm that becomes recognizable early in that part of the embryonic primordium destined to form the rostral region of the head of the vertebrate embryo, and with which the primordium of the notochord soon becomes continuous (Hill and Tribe, 1924).
The term “prochordal plate” (van Oordt, 1921) has been used above as a synonym for the inadvisable term “protochordal plate” (of Hubrecht), but it is also commonly employed (Gilbert, 1957) for a more restricted area known as the prechordal plate (Praechordalplatte of Oppel). Another and unjustified term sometimes found is the “completion plate of the head process” (Ergänzungsplatte des Urdarmstranges of Bonnet). The prochordal plate, in its wider sense, probably does not contribute to the notochord but gives origin to (1) cephalic mesenchyme, (2) at least a part of the lining of the foregut (all or a portion of the endodermal layer of the oropharyngeal membrane), (3) the preoral gut (Seessel’s pocket), and (4) the prechordal plate in a restricted sense (Hill and Tribe, 1924). The production of mesenchyme by the prochordal plate in the human reaches its maximum only after the differentiation of the somites (Hill and Florian, 1931b).
In Tarsius (Hill and Florian, 1963) at a certain stage of development, the thickened endoderm becomes arranged as an “annular zone,” the rostral part of which is constituted by the “prochordal complex” (prochordal plate of earlier stages). In the rabbit, on the other hand, a horseshoe-shaped zone of thickened endoderm has been found, and whether or not it corresponds with the rostral part of Hubrecht’s annular zone remains an open question (Aasar, 1931). In the human also, a horseshoe-shaped band of thickened endoderm was detected by Hill and Florian (1931b) in some embryos of stages 6 (Beneke), 7 (Manchester 1285) and 8 (Thompson-Brash; Dobbin). Either prechordal or prochordal, as used in a purely topographical sense, would seem to be a suitable term in the human embryo, and the former is employed in this study.
In most embryos of stage 8 the prechordal plate has been recorded as present, and it reaches its height of development (up to eight rows of cells) at that stage (O’Rahilly and Müller, 1981). It varies in rostrocaudal length from 0.03 to 0.3 mm. In No. 5960, for example, the plate (fig. 8-8) presents a very irregular dorsal surface “since cells are being given off to the surrounding tissue and some of them should no doubt be classified as mesodermal cells” (Heuser, 1932b).
Chromatophil granules (as described by Bonnet) and small, isolated cavities, or perhaps even a channel (Odgers, 1941; George, 1942), may be found in the plate. Although the prechordal plate meets the notochordal process caudally, it does not ordinarily reach the rostral margin of the embryonic disc. Further detailed studies of the plate in the human embryo, however, are needed (Gilbert, 1957). The difficulties encountered in distinguishing and delimiting the prechordal plate can be seen from the fact that, in the case of one embryo (Manchester 1285), Hill and Florian changed their view concerning its limits and, in the case of a second specimen (No. 5960), these experts disagreed with another (Heuser) concerning its location.
The possible relationship of the prechordal plate to certain types of tumors, such as epignathus, was mentioned by Adelmann (1922). Defects in the prechordal plate may possibly result in holoprosencephaly or agenesis of the corpus callosum (Jellinger et al., 1981).
In summary, the delimitation and first appearance Page 74 of the prechordal plate in the human embryo are not yet clear. It has been found in most embryos of stage 8, in some of stage 7 and even stage 6, and the possibility that it may be present at stage 5c has been raised.
Umbilical vesicle. The cavity of the umbilical vesicle is larger than that of the amnion, and the sac may project beyond the embryonic disc (No. 7640) or be approximately flush with it (Kl.13, No. 8820). The former relationship, however, is considered to be a distortion (George, 1942). Blood islands and blood vessels are seen in the wall of the umbilical vesicle, and an occasional cyst can also be found. Mesenchymal cells, hemocytoblasts, and primitive erythroblasts have been observed in the wall of the umbilical vesicle (No. 8820: Bloom and Bartelmez, 1940). A diverticulum of the vesicle may be present (Kl.13).
A rostrally situated fold of the umbilical vesicle (No. 5960) should not be mistaken for a precocious foregut. In more-advanced specimens (M’Intyre, Gläveke), which may, however, belong to stage 9, an indication of a foregut has been identified. A possible hindgut may be present in Peh. 1-Hochstetter (Florian, 1934b), but the assertion is not particularly convincing.
Primordial germ cells are probably not identifiable from the beginning of development, and probably arise from any of the totipotent or pluripotent cells of the early (e.g., 8-cell) embryo. The details of the arrival of the P.G.C. in the wall of the umbilical vesicle (perhaps by stage 8 and certainly by stage 11) are unknown.
In Bi 24, certain cells in the endoderm in the region of the cloacal membrane, as well as caudally in the endoderm of the umbilical vesicle, were thought to be probably primordial germ cells (Florian, 1931; Politzer, 1933). Such cells, although sought, were not found in some other specimens, however.
Cloacal membrane. The cloacal membrane varies from 0.02 to 0.185 mm in length. It has been suggested that “the cloacal membrane is early more extensive and that it later breaks down at the caudal end where the allantois is fused with the amniotic ectoderm” (Heuser, 1932b). The primitive groove may extend onto the cloacal membrane, as in the Dobbin embryo (Hill and Florian, 1931b).
Allantoic diverticulum (fig. 8-4). Either an allantoenteric (No. 5960, Peh. 1-Hochstetter) or an allantoic (No. 8820, Western Reserve 1) diverticulum may be present. It varies in length from 0.14 to 0.65 mm. Vacuoles may be found in the cells of the rostral portion of the allantoic canal, and have been recorded there especially in later stages. The tip of the allantoic diverticulum may appear as a terminal vesicle and may even be separated from the remainder (Hill and Florian, 1931b).
Connecting stalk. The cells of the stalk are loosely arranged except in the proximity of the allantoic diverticulum. The stalk is covered by a layer of mesothelium, which forms thin-walled elevations that may be mistaken for blood vessels (Bremer, 1914) as may also certain vesicles within the stalk (Hill and Florian, 1931b). Because the vascular network comes in contact with the surface mesothelium in some areas, the latter may be a source, although not a very important one, of vascular endothelium (Heuser, 1932b). In other words, the mesothelium of the connecting stalk may possibly play some part in the formation of vessels (M’Intyre, 1926; but see also Hertig, 1935). Blood vessels are found in the wall of the umbilical vesicle, in the connecting stalk, and in the chorion and villi. Two anastomosing vessels (in Dobbin), one on each side of the allantoic diverticulum, are regarded as the primordia of the umbilical arteries, and a possible representative of the later primordium of the umbilical vein may be present (Hill and Florian, 1931b). The future umbilical arteries are the first channels that can be identified as vessels having a recognizable course (M’Intyre, 1926).
SPECIMENS OF STAGE 8 ALREADY DESCRIBED
Pha XVII. Chorionic cavity, 3.317 x 2.79 x 0.714mm. Embryonic disc, 0.412 mm. This embryo is said to resemble No. 7802 (stage 7). In addition to a notochordal process (Mazanec, 1959, fig. 104) of 0.01 mm, however, it is thought to show probably the Anlage of the notochordal canal and an unusually large prechordal plate. Possible primordial germ cells were seen in the wall of the umbilical vesicle. Presumed age, 16-17 days. Median projection published (ibid., fig. 44).
Carnegie No. 8820, Jones-Brewer I. Described by Jones and Brewer (1941). Hysterectomy. Chorionic cavity, 6 x 5 x 2.5 mm. Embryonic disc (broad type), 0.58 x 0.78 mm (in straight line); 0.6 x 0.79 mm (over curve). Primitive streak, 0.22 mm. Primitive node (0.06 mm) situated somewhat rostral to midpoint of embryonic disc. Three small, discontinuous cavities in node “represent the beginning of a neurenteric canal” which has no dorsal opening and does not communicate with the umbilical vesicle. Notochordal process, 0.0414 mm, but with no canalization. Hemocytoblasts and primitive erythroblasts identified in wall of umbilical vesicle (Bloom and Bartelmez, 1940). Presumed age, 18½ days. Dorsal and median projections published (Jones and Brewer, 1941, figs. 11 and 12; Mazanec, 1959, fig. 48).
Page 75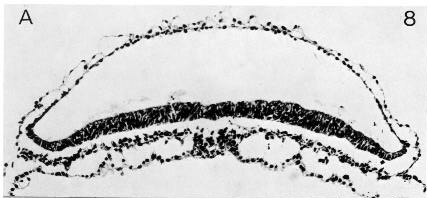
Fig. 8-8. The prechordal plate (Heuser’s interpretation) of No. 5960.
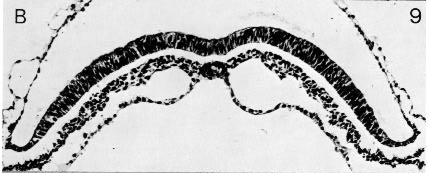
Fig. 8-9. The notochordal plate of No. 5960. The developing neural groove can be seen.
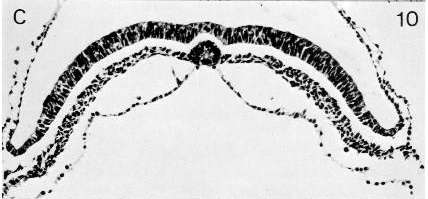
Fig. 8-10. The notochordal process and canal of No. 5960.
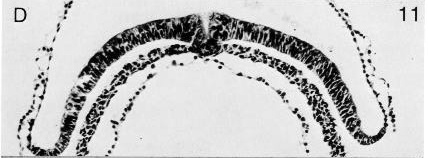
Fig. 8-11. The primitive pit and node of No. 5960.

Fig. 8-12. The primitive streak and groove of No. 5960.
Page 76Carnegie No. 9286 Embryonic disc, 1.13 x 0.77 mm. Primitive streak, 0.38 mm. Notochordal process, 0.15 mm. An excellent specimen reconstructed by O'Rahilly and Müller (1981, fig. 3), who give details of ten other Carnegie specimens
Carnegie No. 9009. Described briefly in an abstract by Heuser (1954). Hysterectomy. Monozygotic twin embryos. Embryonic discs, 0.9 and 0.66 mm. In each: primitive node in middle of disc, notochordal process with first evidence of canalization. Notochordal processes, 0.16 and 0.07 mm. Assigned to horizon VIII by Heuser, who estimated the age as 17 days. Reconstructed by O’Rahilly and Müller (1981, fig. 2B,C).
Shaw. Described by Gladstone and Hamilton (1941). Hysterectomy. Chorion, 11 x 4.04 mm. Chorionic cavity, 8 x 3 mm. Embryonic disc (broad type), 1.05 x 1.34 mm. Notochordal process, 0.17 mm. Primitive pit and notochordal canal (which does not open into the umbilical vesicle). Prechordal plate identified but doubted by Mazanec (1959). No neural groove. Possible amniotic duct. Hemopoiesis (hemocytoblasts and primitive erythroblasts) under way in wall of umbilical vesicle and in connecting stalk. Chorionic villi and endometrium described by Hamilton and Gladstone (1942); trophoblast further described by Hamilton and Boyd (1960). Presumed age, 18 days. Median projection published (Gladstone and Hamilton, 1941; Mazanec, 1959, fig. 58).
Wa 17 (Wagner). Described by Grosser (1931a,b). Hysterectomy. Chorionic cavity, 8.5 x 8.5 x 7.5 mm. Embryonic disc (narrow type), 0.98 x 0.7 mm. Primitive streak, 0.5 mm. Notochordal process, 0.18 mm. Possible dorsal and ventral openings of the notochordal canal. Prechordal plate, 0.075 mm. Presumed age, about 19 days. Dorsal and median projections published (Grosser, 1931a, figs. 4 and 3; Hill and Florian, 1931b, fig. 7; Mazanec, 1959, fig. 59).
Carnegie No. 8671. Low-power photomicrographs reproduced by Hertig (1968, figs. 47 and 181). Notochordal process, 0.23 mm.
Kl. 13. Described by Grosser (1913). Traumatic abortion following salpingo-oophorectomy. Chorionic cavity, 8 x 6 mm. Embryonic disc, 0.67 x 0.5 mm. Primitive streak, 0.27 mm. Notochordal process, 0.2 mm. Notochordal canal, 0.25 mm (Florian, 1934c) with dorsal pit and ventral opening: notochordal plate intercalated in endoderm. Possible prechordal plate. Presumed age, about 18 days. Median projection published (Grosser, 1913, plate 27, fig. 4; Mazanec, 1959, fig. 62). Compared with other specimens by Grosser (1934).
HEB-42. Described by Mazanec and Musilovà (1959). Curettage. Embryonic disc, 1.17 x 0.72 mm; 1.43 mm by flexible scale. Primitive streak, 0.54 mm. Primitive node, 0.06 mm. Primitive pit. Notochordal process, 0.25 mm. Small cavity (primordium of notochordal canal) in notochordal process. Prechordal plate not mentioned. Presumed age, 17-18 days. Dorsal and median projections published (ibid., figs. 1 and 2).
Dy (Dyhrenfurth). Described by Triepel (1916). Abortion. Embryonic disc, 1.6 x 1.04 mm. Primitive streak, 0.11 mm. Notochordal process and plate, 0.3 mm. Primitive pit, neurenteric canal, and neural groove believed to be present. Anlagen of hypophysis and optic vesicles claimed unconvincingly (plane of section unsuitable). Embryonic disc bent ventrally through a right angle (normality of specimen questioned). First somite probably not present.
Thompson and Brash (1923) described a hysterectomy specimen that showed a chorionic cavity of 10 x 7.5 x 4 mm. Embryonic disc (broad type), 0.68 x 0.9 mm. Primitive streak and groove present. Notochordal process, 0.3 mm, with no distinct lumen but notochordal canal about to appear. Mazanec (1959) considered that, on the basis of the reconstruction, the notochordal process could not be more than 0.23 mm. Definite prechordal plate (Hill and Florian, 1931b). Rough dorsal and median drawings included (Thompson and Brash, 1923, figs, 2 and 3) and median projection published (Mazanec, 1959, fig. 55). This embryo belongs either to stage 7 or to stage 8.
Schö (Schönholz). Described by Waldeyer (1929a,b). Hysterectomy. Embryonic disc, 0.99 x 1.03 x 0.11 mm. Primitive streak, 0.51 mm, and node. Primitive groove and pit: “indentation” is perhaps “first Anlage of [notochordal] canal.” Notochordal process, 0.34 mm. Prechordal plate. Said to lie between Hugo (stage 7) and Peh. l-Hochstetter (stage 8). Dorsal and median projections published (ibid., figs. 6 and 5; Hill and Florian, 1931b, figs. 6 and 14; Mazanec, 1959, fig. 57).
Dobbin. Important specimen described in detail by Hill and Florian (1931a,b,c). Abortion. Chorion, 11.5 x 8.5 x 4.5 mm. Chorionic cavity, 9 x 5.5 x 2.5 mm. Embryonic disc (narrow type), 0.96 x 0.41 mm. Primitive streak, 0.42 mm to notopore. Notochordal process, 0.42 mm. Notochordal canal communicates with cavity of umbilical vesicle by seven openings, the caudalmost of which is the ventral opening of a very short neurenteric canal. Prechordal plate, 0.03 mm; rostral end of notochordal process was at first mistaken for prechordal plate. Dorsal and median projections published (Hill and Florian, 1931b, figs. 1 and 2; Mazanec, 1959, fig. 60). The scale in fig. 3 of Hill and Florian (1931b) is incorrect (Florian, 1934c). Specimen is now housed in Hubrecht Laboratory, Utrecht (No. H91; HH 159).
Page 77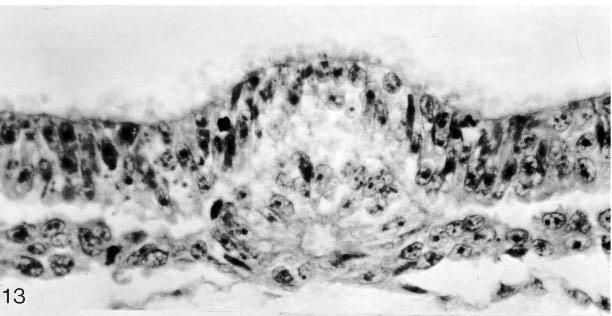
Fig. 8-13. The notochordal process and notochordal canal of No. 7545. Section 4-2-14. Cf. fig. 8-18.
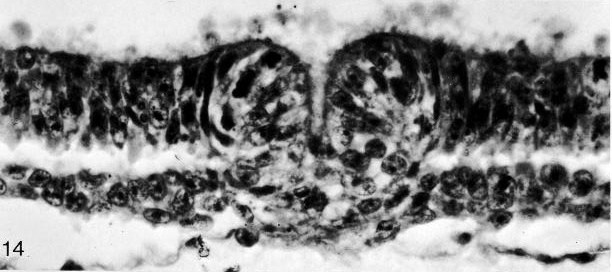
Fig. 8-14. The primitive pit of No. 7545. Section 4-3-7. Cf. fig. 8-19.
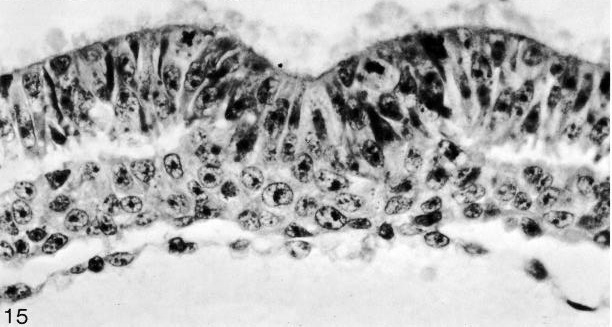
Fig. 8-15. The primitive groove and primitive streak of No. 7545. Section 5-2-9.
Page 78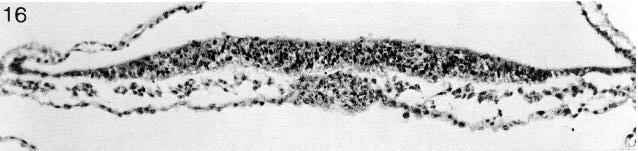
Fig. 8-16. The prechordal plate of No. 7545. Section 2-4-13.

Fig. 8-17. The neural groove of No. 7545. Section 3-3-9.
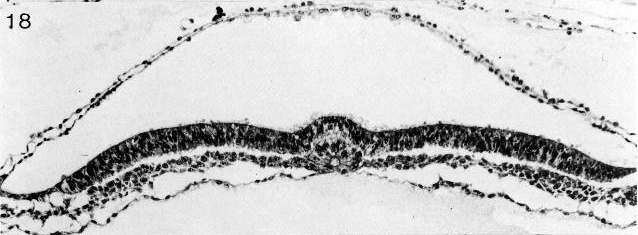
Fig. 8-18. The notochordal process and notochordal canal of No. 7545. Section 4-2-14. Cf. fig. 8- 13.
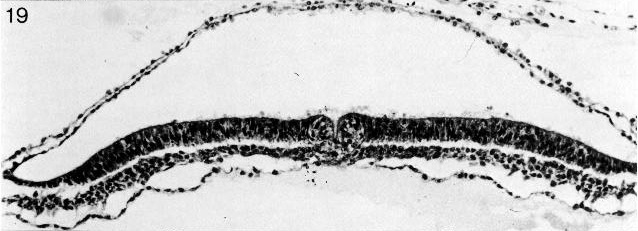
Fig. 8-19. The primitive pit of No. 7545. Section 4-3-7. Cf. fig. 8-14.
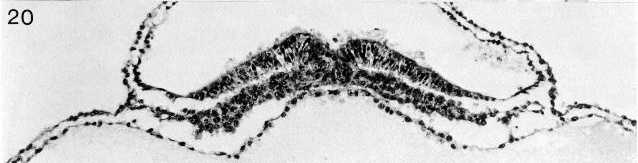
Fig. 8-20. The primitive groove and primitive streak of No. 7545. Section 6-1-2.
Page 79Carnegie No. 5960 (figs. 8-3 to 8-12). Important specimen described by Heuser (1932b). Hysterectomy. Chorion, 15 x 14 x 9 mm. Embryonic disc (narrow type), 1.25 x 0.68 mm (in straight line); 1.53 x 0.75 mm (by flexible scale). Primitive streak, 0.5 (0.44?) mm. Primitive node, 0.2 (0.06?) mm, slightly caudal to midpoint of embryonic disc. Notochordal process, 0.42 mm (George, 1942). Notochordal canal (about 0.4 mm) opens ventrally. Prechordal plate, 0.15 mm. Florian (1934b), however, believed that the prechordal plate was situated further rostrally than shown by Heuser. Angiogenesis in umbilical vesicle, body stalk, and chorion. Angiogenesis in chorion described by Hertig (1935). Neural groove. Presumed age, 18 days. Dorsal and median projections published (Heuser, 1932b, figs. 33 and 47; Mazanec, 1959, fig. 63).
Carnegie No. 7545 (figs. 8-13, 8-14, 8-15, and 8-16 to 8-20). Embryonic disc, 1.52 x 1.03 mm. Primitive streak, 0.61 mm. Notochordal process, 0.43 mm. Reconstructed by O'Rahilly and Müller (1981, fig. 2E).
Carnegie No. 7640. Described by George (1942). Tubal. Embryonic disc (broad type), 1.01 x 0.83 mm (in straight line); 1.16 mm by flexible scale. Primitive streak, node, and pit present. Notochordal process, 0.44 mm. Notochordal canal continuous with primitive pit; floor of canal has disappeared in its middle quarter. Prechordal plate, 0.12 mm, said to contain continuation of notochordal canal. Neural groove. Dorsal and median projections published (ibid., figs. 1 and 2).
Peh. 1-Hochstetter (Peham). Described by Rossenbeck (1923). Chorion, 10 x 7.7 mm. Chorionic cavity, 6.8 x 5.3 mm. Embryonic disc (broad type; this might be disputed, however), 1.77 (Florian, 1934) x 1 mm. Primitive streak, 0.69 mm. Notochordal process (Mazanec, 1959, figs. 107 and 108), 0.6 mm. Notochordal canal ready to break through into umbilical vesicle in one section. Prechordal plate (confirmed by Florian, 1931), 0.08 mm. Indication of hindgut (Florian, 1934b). Allanto-enteric diverticulum (Florian, 1930a). Dorsal and median projections published (Hill and Florian, 1931b, figs. 9 and 16; Mazanec, 1959, fig. 61).
R. S. (Robb Smith). Described by Odgers (1941). Hysterectomy. Embryonic disc (broad type), 1.5 x 1.36 mm. Primitive streak, 0.4 mm. Notochordal plate (intercalated in endoderm), 0.7 mm. Neurenteric canal extends vertically from amniotic cavity to umbilical vesicle. Prechordal plate, 0.29 mm, containing perhaps remains of notochordal canal. Commencing neural groove. Dorsal and median projections published (ibid., figs. 1 and 2).
Western Reserve No. 1. Described by Ingalls (1918). Abortion. Chorion, 9.1 x 8.2 x 6.5 mm. Chorionic cavity, 8 x 7 x 5 mm. Embryonic disc (narrow type), 2 (1.87?) x 0.75 mm. Primitive streak, 0.67 mm. Primitive pit present. Notochordal process, 0.75 (0.65?), Hill and Florian, 1931b) mm. Notochordal canal, 0.34 mm, with three ventral openings into umbilical vesicle. Prechordal plate identified (but not 0.4 mm in length, according to Mazanec, 1959). Dorsal and median projections published (Hill and Florian, 1931b, figs. 10 and 17; Mazanec, 1959, fig. 64).
ADDITIONAL SPECIMENS
Precise measurements of the notochordal process have not been provided in the accounts of the following embryos.
M’lntyre. Described by Bryce (1924) and M’Intyre (1926). Hysterectomy. Chorion, 14 x 13 x 8 mm. Embryonic disc, 1.37 x 0.5 mm. Primitive streak, 0.32 mm. Notochordal plate, neurenteric canal, and prechordal plate (with cavity) present. Neural groove, future foregut, U-shaped pericardial cavity, and “some general resemblance to somites” noted. Rough dorsal and median drawings included (Bryce, 1924, figs. 5 and 49). May belong to stage 9.
Frassi’s specimen (Keibel, 1907; Frassi, 1908) was illustrated as Normentafel No. 1 by Keibel and Elze (1908). Embryonic disc, 1.17 x 0.6 mm. Primitive streak and neurenteric canal identified. Neural groove. No somites.
Gle., or Gläveke (von Spee, 1889 and 1896). Illustrated as Normentafel No. 2 by Keibel and Elze (1908). See also Kollmann (1907) and Keibel and Mall (1910, 1912). Chorion, 6 x 4.5 mm. Chorionic cavity, 5.3 x 3.8 mm. Embryonic disc, 1.54 mm. Primitive streak and node, notochordal plate, and Page 80 neurenteric canal (Van Beneden, 1899) identified (Keibel and Mall, 1912, fig. 231). Neural groove. Indication of foregut and pericardial cavities. Anlage of endocardium. No somites detected, although a small cavity on the left side (Keibel and Elze, 1908, fig. 4f) could be considered as the first Anlage of a myocoele. May belong to stage 9.
Strahl (1916) described briefly a specimen that possessed a notochordal process and canal, and apparently a prechordal plate. A median drawing was included (ibid., fig. a) but measurements were not provided.
Vuill., or Vulliet. Illustrated schematically by Eternod (1899a and 1909), Kollmann (1907), and Keibel and Mall (1912). Chorion, 10 x 8.2 x 6 mm. Chorionic cavity, 9 x 7.2 x 5 mm. Embryonic disc, 1.3 mm. Notochordal and neurenteric canals (Eternod, 1899b).
Cordier and Coujard (1939) described an embryo of 1.05 mm showing a neural groove and folds, notochordal canal, primordial germ cells, but no somites, no intra-embryonic coelom, and no cardiac rudiment.
Carnegie No. 8727. Photomicrograph reproduced by Hertig (1968, fig. 180). The partial duplication of the embryonic disc shown in this specimen would presumably have resulted in conjoined twins.
Certain other embryos that probably belong to stage 8 but that have not been described in adequate detail will not be referred to here. These include Krukenberg (1922) and Fitzgerald- Brewer I (Brewer and Fitzgerald, 1937). Boerner-Patzelt and Schwarzacher (1923) described an unsatisfactory specimen (embryonic disc, 0.47 x 0.43 mm) that showed a neurenteric canal, Broman (1936) described in detail an abnormal specimen (“Lqt”) in which the primitive streak showed “overgrowth” in relation to the neurenteric canal, resulting in a dislocation within the embryonic disc.
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
