Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 3
Page 17Approximately 0.1 - 0.2 mm in diameter
Approximately 4 postovulatory days
Characteristic feature: free blastocyst
Stage 3 consists of the free (that is, unattached) blastocyst, a term used as soon as a cavity (the blastocystic, or segmentation, cavity) can be recognized by light microscopy. (The staging system is based on light microscopy and, in later stages, on gross structure also.)
The blastocyst is the hollow mass of cells from the initial appearance of the cavity (stage 3) to immediately before the completion of implantation at a subsequent stage. The blastocystic cavity, under the light microscope, begins by the coalescence of intercellular spaces when the organism has acquired about 32 cells. In in vitro studies, a cavity formed in some human embryos at 16-20 cells (Edwards, 1972).
It is necessary to stress that the cavity of the mammalian blastocyst is not the counterpart of the amphibian or the avian blastocoel. In the bird, the blastocoel is the limited space between the epiblast and the primary endoderm. The cavity of the mammalian blastocyst, however, corresponds to the subgerminal space together with the area occupied by the yolk (Torrey, personal communication, 1972).
The mammalian blastocyst differs from a blastula in that its cells have already differentiated into at least two types: trophoblastic and embryonic cells proper.
Heuser and Streeter (1941) emphasized an important point by using stage 3 as an example:
The blastocyst form is not to be thought of solely in terms of the next succeeding stage in development. It is to be remembered that at all stages the embryo is a living organism, that is, it is a going concern with adequate mechanisms for its maintenance as of that time.
It is no less true, however, that changes occur "in the growing organism and its environment which provide critically for the future survival of the organism" (Reynolds, 1954). Indeed, such morphological and functional changes during development "critically anticipate future morphological and functional requirements for the survival and welfare of the organism" (ibid.).
Sex chromatin has been "tentatively identified" in two in vitro human blastocysts (Edwards, 1972).
Probably the first recognition of the inner cell mass of the mammalian (dog and rabbit) blastocyst was that by Prévost and Dumas in 1824. This and many other aspects of the blastocyst are considered in a book edited by Blandau (1971).
SIZE AND AGE
In the human embryo the maximum diameter increases from 100-200 μm at stages 2 and 3 to 300-450 μm at stage 5a.
Embryos of stage 3 are believed to be about 4 days in age. In vitro embryos of stage 1 have been recorded at 9-32 hours after insemination; stage 2 at 22-40 hours (2 cells), 32-45 hours (4 cells), and 48 hours (8 cells); stage 3 at 100 hours, and extruding from the zona pellucida at 140-160 hours, at which time they show differentiation into trophoblast, epiblast, and hypoblast (Mohr and Trounson, 1984).
HISTOLOGICAL FEATURES
Zona pellucida. In stage 3 the zona pellucida may be either present or absent. In vitro, the blastocyst emerges from the zona at about 6-7 days. The emergence is commonly referred to as "hatching."
Trophoblast. During stage 3 the trophoblastic cells, because of their peripheral position, are distinguishable from the embryonic cells proper. The trophoblastic cells that cover the inner cell mass are referred to as polar: i.e., at the embryonic pole or future site of implantation. The remaining trophoblast is termed mural.
Cavitation. It is believed that the blastomeres (in the mouse) attain the ability to secrete the blastocystic fluid after a definite number of cleavages, namely at the end of the fifth and at the beginning of the sixth Page 18 mitotic cycle. In the mouse it has been shown that, when the organism consists of about 32 cells, small cavities unite to form the beginning of the blastocystic cavity. In other words, the solid phase of development (in the mouse) ends at about 28-32 cells, when fluid begins to accumulate beneath the trophoblastic cells. As the blastocyst develops, it undergoes expansions and contractions. When contracted, a "pseudomorula" of about 100 cells in the mouse can be seen.
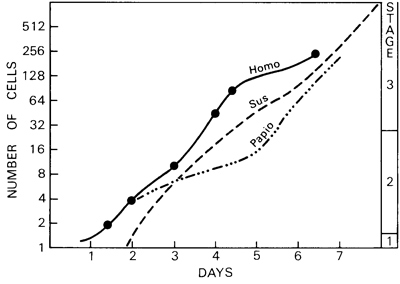
Fig. 3-1. Graph showing presumed age plotted against number of cells. The continuous line is based on six human embryos: 8698, Doyle etai, 8904, 8794, 8663, and Krafka. The interrupted lines indicate pig (Heuser and Streeter, 1929) and baboon (Hendrickx, 1971) embryos. In each case the rate of cleavage during the first week is not much faster than one division per day.
Because no appreciable increase in size of the (cat) embryo occurs at first, it is thought that no mere flowing together of inter- or intra-cellular spaces or vacuoles is a sufficient explanation of the origin of the blastocystic cavity. Thus an additional factor, namely cytolysis of certain of the central cells, is also involved.
Electron microscopy has added further details. The formation of junctional complexes, which is regarded as the first sign of blastocystic formation, is found very early in the rat, when the embryo consists of only 8 cells, although the first indication of a cavity, as opposed to intercellular spaces, is not seen until after another series of cell divisions. In two human, 8-cell in vitro embryos studied by electron microscopy, "a small cleavage cavity was already apparent within each embryo" (Sathananthan, Wood, and Leeton, 1982).
Inner cell mass. The embryonic cells proper become surrounded by the trophoblastic cells and form an inner mass. Studies of various mammals have indicated that the inner cell mass represents more than the embryo itself, insofar as it constitutes a germinal mass of various potentialities which continues for a time to add cells to the more precociously developed trophoblast. The inner cell mass gives origin to the hypoblast, and its remainder (the "formative cells") constitutes the epiblast. The epiblastic cells soon become aligned into what wras frequently described as the "germ disc." These various relationships are summarized in figure 6-2, It has been found that hypoblastic differentiation in the macaque occurred at about the same time that a basal lamina was found under mural trophoblast and epiblast (but not polar trophoblast or hypoblast) (Endersand Schlafke, 1981).
Duplication of the inner cell mass probably accounts for most instances of monozygotic twinning (Corner, 195S; Bulmer, 1970). Such twins should be monochorial but diamniotic (fig. 5-2). In vitro, "many blastocysts fail to hatch fully from their zona pellucida," and "two separate emhryos could form if the inner Page 19 cell mass was bisected during hatching" (Edwards, Mettler, and Walters, 1986).
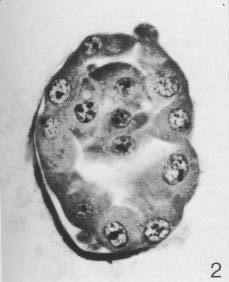
Fig. 3-2. Section through a 58-cell blastocyst (No. 8794). The zona pellucida is visible on the lower left-hand part of the mass, where a polar body can also be recognized. The inner cell mass can be seen above the blastocystic cavity. The more peripherally situated cells are trophoblastic.
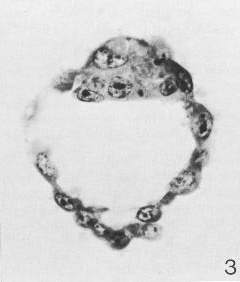
Fig. 3-3. Section through a 107-cell blastocyst (No. 8663). The zona pellucida is no longer present. The blastocystic cavity is now quite large. The embryonic pole, characterized by the inner cell mass, is shown uppermost. The peripheral layer of cells constitutes the trophoblast.
Two significant specimens of stage 3 (Hertig etal, 1954) are here cited.
In a 58-cell specimen (No. 8794), 53 of the cells were trophoblastic whereas 5 were embryonic. The latter composed the inner cell mass, which was located eccentrically within the blastocystic cavity but had not yet assumed a truly polar position. In a 107-cell specimen (No. 8663), 99 of the cells were trophoblastic, and, of these, 69 were mural in position and 30 were polar (i.e., covering the embryonic pole). Eight of the 107 cells were embryonic, and were characterized by their larger size and by the presence of intracytoplasmic vacuoles. Moreover, the 8 cells comprised three types: "obvious primitive, vacuolated ectoderm [epiblast]; flattened primitive endoderm [hypoblast]; and a large indifferent cell, presumably a [primordial] germ cell" (Hertig, 1968). In addition, of the 30 polar trophoblastic cells, 4 which were situated "ventral and lateral to the formative cells ... may actually be of primitive endodermal type" (Hertig etal, 1954).
Dorsoventrality. A comparison of stage 3 embryos with those of stage 5 makes it clear that the surface of the inner cell mass that is adjacent to the polar trophoblast represents the dorsal surface of the embryo, and the surface of the mass that faces the blastocystic cavity represents the ventral surface. In other words, "dorsalization," or "dorsoventrality," becomes apparent during stage 3 (O'Rahilly, 1970). The possibility should be kept in mind, however, that the inner cell mass can perhaps travel around the inside of the trophoblastic layer.
Rate of division. In the pig embryo it has been shown that, in general, "'during the first seven days the cells undergo about eight divisions, that is, they divide about once a day" (Heuser and Streeter, 1929). A similar generalization may be made for the human embryo during stages 1-3, and also for the baboon (Hendrickx, 1971). In the case of the baboon, "there is a close correlation between age and cell number," although "there is no consistent relationship between age and size for these stages of development" (ibid.).
Page 20SPECIMENS OF STAGE 3 ALREADY DESCRIBED
Ca. 32 cells. Described by Shettles (1956 and 1960). Produced in vitro. Zona pellucida denuded of corona and cumulus cells. "Early segmentation cavity."
Ca. 50 cells. Described by Shettles (1957) as caused by "parthenogenetic cleavage." Zona pellucida denuded of corona and cumulus cells. Diameter, including zona, 150 urn. "Early segmentation cavity."
58 cells, Carnegie No. 8794. Described by Hertig etal. (1954). Uterine. Diameter, 230 x 190 |xm (108 x 86 after fixation; 101 x 73.3 after sectioning); diameters of blastomeres varied from 15 to 23 after sectioning; polar bodies, 18 urn after fixation. Zona pellucida intact while fresh but partly deficient after fixation (fig. 3-2). Two polar bodies. Early blastocystic cavity. Believed to be about 4 days old.
100 cells. Described by Khvatov (1967). Tubal. Diameter, 126 x 100 x 70 (i.m. Nuclei in trophoblastic blastomeres darker (with hematoxylin and eosin). Said to be female, "based on current studies concerning sex chromatin."
"More than 100 cells." In two such blastocysts produced in vitro, "bodies resembling sex chromatin were seen in a few nuclei" (Steptoe, Edwards, and Purdy, 1971; Edwards, 1972).
107 cells, Carnegie No. 8663. Described by Hertig etal. (1954). Uterine. No zona pellucida (fig. 8). Diameter, 153 x 115 urn (103 x 80 after fixation; 91.6 X 83.3 after sectioning); diameters of blastomeres varied from 8 to 21. Large blastocystic cavity (58 urn). Embryonic mass composed of 8 cells: epiblastic, hypoblastic, and a presumed primordial germ cell (Hertig, 1968). Believed to be about 4½ days old. Khvatov (1967), without further elaboration, claimed: "according to photographs, should be of the male sex." Smith (1970, fig. 15), without further justification, claimed that a cytoplasmic vacuole was "the first indication toward an amniotic space."
186 cells. Croxatto etal, (1972) suggested that "the unimplanted human blastocyst begins a process of expansion when it has between 107 and 186 cells," prior to shedding of the zona pellucida.
Ca. 200-300 cells. Described briefly by Krafka (1942). Tubal. Diameter, 120 x 180 urn. Zona pellucida intact. Some adherent granulosa cells. Described as "solid" but the large number of cells suggests that it should have had a blastocystic cavity (it may be a contracted blastocyst); hence it is included here in stage 3.
Unknown number of cells. Three blastocysts that failed to emerge from the zona were studied by electron microscopy (Lopata, Kohlman, and Kellow, 1982). One showed hypoblast.
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
