Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 19
Page 237INTRODUCTION TO STAGES 19-23
As the embryo advances into more-specialized levels, it becomes necessary to take into account the developmental status of various internal structures in order to assign the specimen precisely to a stage.
For stages 19–23, Streeter devised a system of rating the development of selected structures by “point scores.” The eight features chosen were the cornea, optic nerve, cochlear duct, adenohypophysis, vomeronasal organ, submandibular gland, metanephros, and humerus. He regarded these as arbitrarily selected key structures that were readily recognizable under the microscope and that could be recorded by camera lucida sketches from serial sections.
The developmental points for a given embryo were added, and the resulting value was used to determine not only the stage (Table 19-1) but even the relative position within that stage.
Although Streeter’s original data for the point scores for the various features of individual embryos are no longer available, the final score for each embryo that he studied at stages 19–23 is known and appears in the lists of embryos given here.
Table 19-1. Number of Developmental Points per Embryo
| Stage | Range of Points |
|---|---|
| 19 | 10 - 16.5 |
| 20 | 19 - 29.5 |
| 21 | 30 - 39 |
| 22 | 40.5 - 46 |
| 23 | 48 - 60.5 |
A detailed table showing the assignment of scoring points is available in Streeter’s work (1951, pp. 170 and 171) but is not reproduced here because of the considerable technical difficulty that would be involved in repeating Streeter’s successful but rather personal procedure in a consistent manner. Moreover, some slight problems exist in the arrangement of the table, as it was printed. These may have been caused by the circumstance that the final version had to be prepared by others, namely Heuser and Corner.
It is to be stressed that considerable caution needs to be exercised in assigning a given embryo to stages 19–23, particularly in the absence of detailed studies of its internal structure. The external form of a new embryo should be compared in detail with Streeter’s photographs (figs. 19-1, 20-1, etc.). The greatest length of the embryo should also be taken into account.
Stage 19 is not quite as difficult as the later stages to identify, because toe rays are prominent but interdigital notches have not yet appeared. The foot is still an important guide in later stages but difficulties may well be encountered in stages 20–22, such that it is frequently more prudent to conclude with a weak designation such as “approximately stage 20” or “stage 20–21” or “probably stage 22.” Stage 23, at least at its full expression, is again less difficult to identify because of its more mature appearance. In addition, certain internal features, such as the status of the skeleton, are important guides for stages 19–23.
Some embryos from other laboratories have unfortunately been recorded in the literature as belonging to various specific stages without sufficiently detailed and careful examination of their structural features.
Page 238
Fig. 19-1. Photographs of three embryos belonging to stage 19. The trunk is now beginning to straighten, and the cervical angulation is less acute. The coalescence of parts in the pharyngeal region has altered the appearance of the auricle, and the hillocks are less conspicuous. The longitudinal axes of the upper limbs are almost at a right angle to the dorsal contour of the body of the embryo, and the axes of the upper and lower limbs are more or less parallel, so that each limb has a pre-axial and a postaxial border. The transverse and sigmoid sinuses are prominent in D. The brain in E shows, from above downward, the mesencephalon, diencephalon, and cerebral hemispheres. Top row, No. 8092. Middle row, No. 6824. Bottom row, No. 4501. All limbs are at the same magnification.
Page 239STAGE 19
SIZE AND AGE
Most embryos of this stage measure 17–20 mm in length.
The age is believed to be approximately 47–48 postovulatory days. One embryo (No. 1390) is known to be “exactly 47 days” (Mall, 1918), although another appears to have been only 41 days (Moore, Hutchins, and O’Rahilly, 1981).
EXTERNAL FORM
The trunk has begun to elongate and straighten slightly, with the result that the head no longer forms a right angle with the line of the back of the embryo. The limbs extend nearly directly forward. The toe rays are more prominent, but interdigital notches have not yet appeared in the rim of the foot plate.
FEATURES FOR POINT SCORES
1. Cornea. A thin layer of mesenchyme crosses the summit of the cornea (Streeter, 1951, fig. 14). General views of the eye at stages 13–23 are shown in figures 19-2 and 19-3, and at stages 19–23 in figure 19-5.
2. Optic nerve. The optic nerve is slender (fig. 19-4). Nerve fibers can be traced for a short distance beyond the retina but have not yet reached the middle region of the stalk.
3. Cochlear duct. The tip of the L-shaped cochlear duct turns “upward” (fig. 19-6).
4. Adenohypophysis. The pars intermedia is beginning to be established, as well as the lateral lobes that will form the pars tuberalis (fig. 19-7). The stalk is relatively thick and contains the remains of the lumen of the hypophysial sac. Transverse views of the gland at stages 19–23 are shown in figure 19-8.
5. Vomeronasal organ. The vomeronasal organ is a thickening of the nasal epithelium that still bounds a shallow groove or pit (fig. 19-9).
6. Submandibular gland. The primordium of the submandibular gland is present in stage 18 as an epithelial thickening in the groove between the tongue and the future lower jaw. In stage 19, an area of condensed mesenchyme appears beneath the duct (fig. 19-10 and Streeter, 1951, fig. 24).
7. Metanephros. In many areas the metanephrogenic tissue around the ampullae of the collecting tubules remains undifferentiated but, in others, nodules become separated from the metanephrogenic mantle (fig. 19-11). In some of these, small cavities have appeared, transforming them into renal vesicles.
8. Humerus. Differentiation of chondrocytes had already progressed to phase 3 by stage 18, and phases 1–3 continue to be present in stages 19 and 20 (Streeter, 1949, fig. 3 and plate 2). The middle of the shaft is becoming clearer. Although no sign is present in the humerus, ossification begins in the clavicle and mandible in one of the stages from 18 to 20, and in the maxilla at stage 19 or stage 20 (O’Rahilly and Gardner, 1972).
ADDITIONAL FEATURES
Blood vascular system. The arterial system has been depicted by Evans in Keibel and Mall (1912, fig. 447). Excellent views of reconstructions of the aortic arches at stages 11–19 were published by Congdon (1922, figs. 29–40).
Heart. The ventricles are situated ventral to the atria, so that a horizontal section corresponds approximately to a coronal section of the adult organ. Such a section has been reproduced by Cooper and O’Rahilly (1971, fig. 1), who also provided an account of several features of the heart at stages 19–21. Fusion of the aortic and mitral endocardial cushion material occurs at stage 19 (Teal, Moore, and Hutchins, 1986). Page 240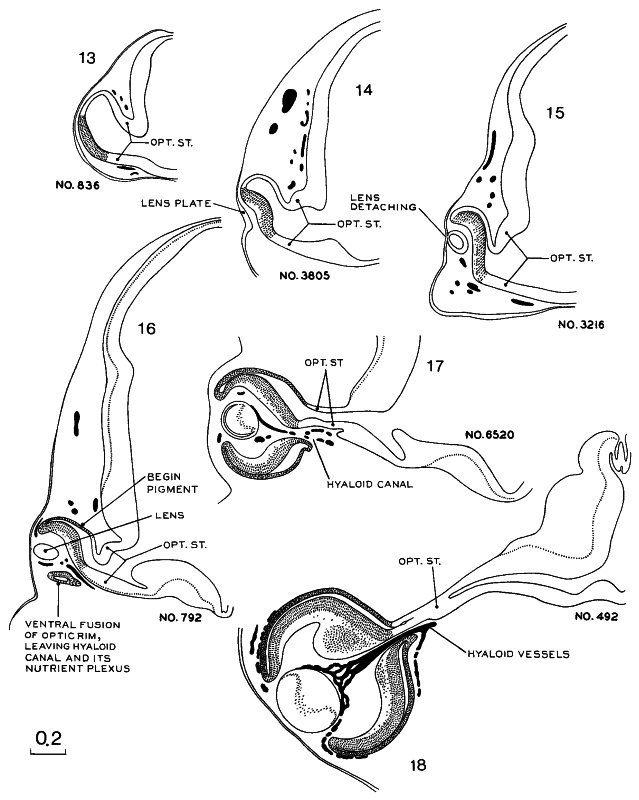
Fig. 19-2. Drawings of sections through the optic vesicle/cup and stalk at stages 13–18. As the optic vesicle is being transformed into the optic cup, the inverted (neural) lamina of the retina retains direct connection at all times with the wall of the brain. At stages 13–17 the optic ventricle communicates freely with the cavity of the diencephalon. The body of the lens is thickening and, in stage 18, the primary fibers fill the lumen of the lens.
Page 241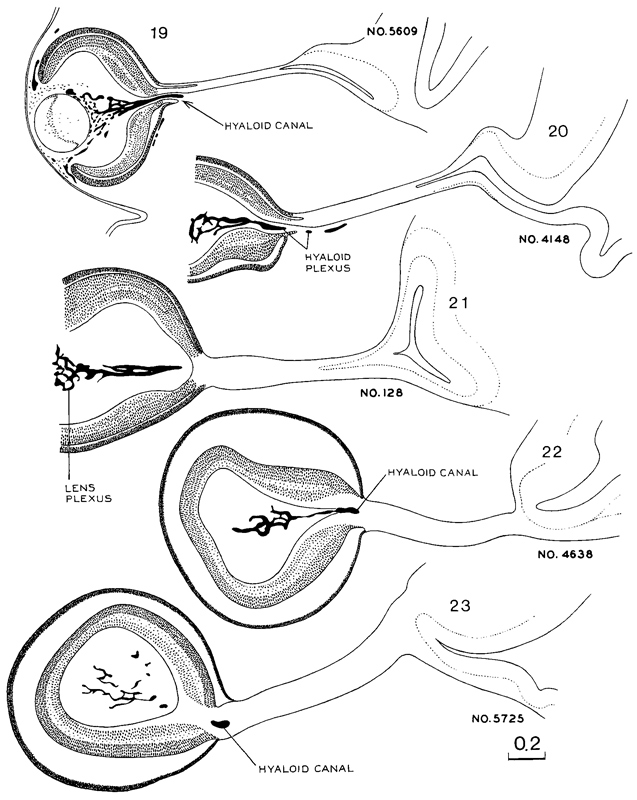
Fig. 19-3. A continuation of the previous illustration, showing the eye and optic nerves at stages 19–23. Several sections in each embryo were combined to show the form of the optic nerve. All drawings in this and the previous figure are at the same scale.
Page 242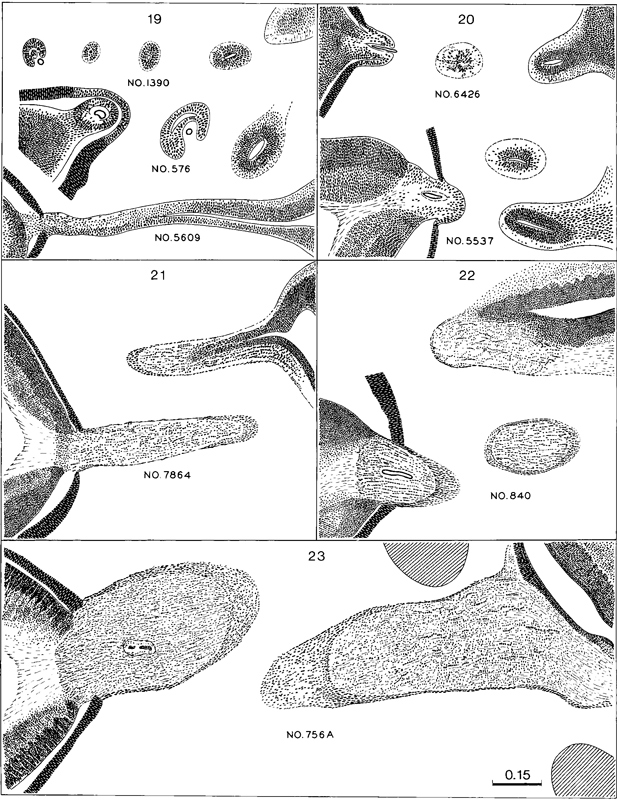
Fig. 19-4. Drawings of the sections showing the form of the optic nerve and the progressive spread of nerve fibers from retina to brain. Some fibers have grown through the whole tract (which really is the so-called optic nerve) and are arriving at the brain at stage 20. In the more advanced stages many (neuro-ectodermal, including glial) cells are seen in longitudinal rows between bundles of nerve fibers. All drawings are at the same scale, which is slightly less than twice that of the previous two figures.
Page 243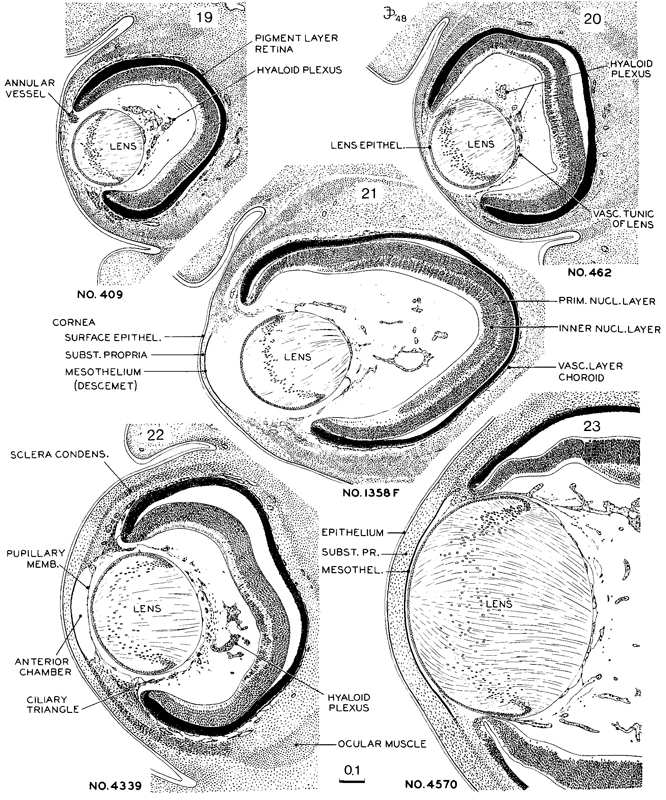
Fig. 19-5. Drawings of mid-sections of the eye at stages 19–23. The sections were selected to demonstrate the organization and relations of the various parts of the eye. The lens remains nearly spherical throughout this period and occupies a relatively large amount of the eye. The pupillary membrane is developing and the anterior chamber is emerging (stage 22). The hyaloid plexus partly fills the vitreous chamber and forms a network on the back of the lens. All drawings are at the same scale.
Page 244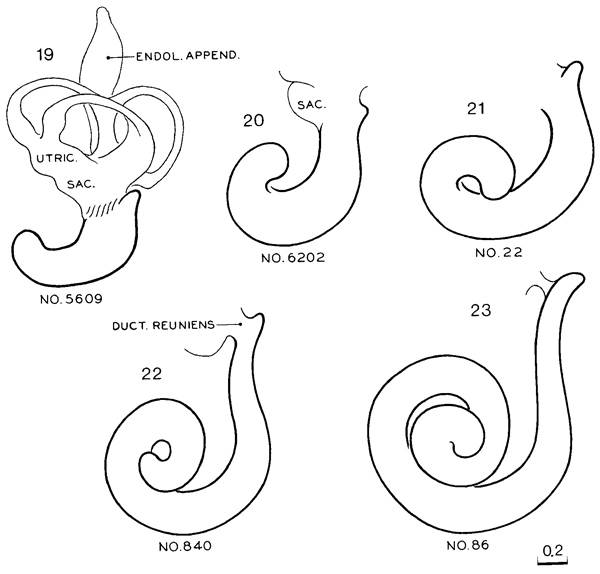
Fig. 19-6. Reconstructions of the cochlear duct at stages 19–23. All drawings are at the same scale. The position of the membranous labyrinth in situ has been shown by O’Rahilly and Gardner (1971, fig. 1) at stage 21 and by Streeter (1906, fig. 2) at stage 23. The relationship of the membranous labyrinth to the otic capsule at stage 23 has been illustrated by Müller and O’Rahilly (1980b, fig. 5).
Lungs. The bronchial tree was reconstructed by Wells and Boyden (1954, plates 3 and 4). The first generation of subsegmental bronchi is now complete.
Cloacal region. The cloacal membrane ruptures from urinary pressure at stage 18 or stage 19, and the anal membrane becomes defined.
Gonads. The rete testis develops from the seminiferous cords at stages 19–23, and the tunica albuginea forms (Jirasek, 1971). Cords representing the rete ovarii are also developing (Wilson, 1926a).
Sternum. Right and left sternal bars, which are joined cranially to form the episternal cartilage, are present by stage 19 (Gasser, 1975, figs. 7-17 and 7-22).
Brain A general view of the organ was given by Gilbert (19S"7, fig. 18), and certain internal details were provided by Hines (1922, figs. 15 and 16).
In the rhombencephalon the migration for the formation of the olivary and arcuate nuclei has begun. In most embryos the choroid plexus of the fourth ventricle is now present. In median sections the paraphysis marks the limit between diencephalic and telencephalic roof. A characteristic wedge appearance of the "anterior lobe" of the epiphysis is found (O'Rahilly, 1968). The stria medullaris thalami reaches the habenular nuclei. The habenular commissure may begin to develop. Half of the length of the cerebral hemispheres reaches further rostrally than the lamina terminalis, and half of the diencephalon is covered by them. The occipital pole of the hemisphere can be distinguished caudally and ventrolaterally. The internal capsule begins to develop.
EMBRYO OF STAGE 19 ALREADY DESCRIBED
Harvard No. 839, 17.8 mm G.L. Described and illustrated in monographic form by Thyng (1914).
Page 245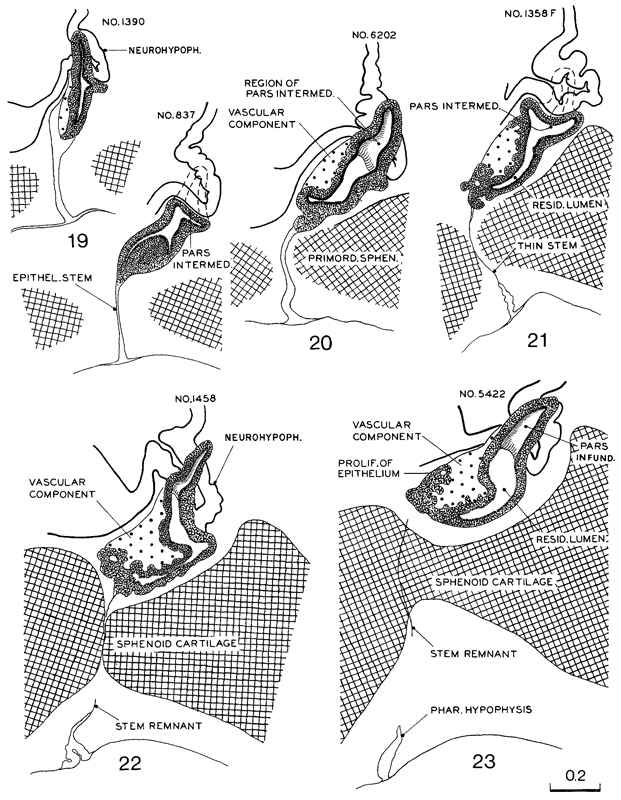
Fig. 19-7. Median sections of the hypophysis cerebri at stages 19–23. Two or more sections were combined in preparing the drawings. Several sections were used to represent the lateral processes of the pars intermedia, which grow dorsally around the sides of the infundibular stem to form the pars infundibularis (or tuberalis). All drawings are at the same scale.
Page 246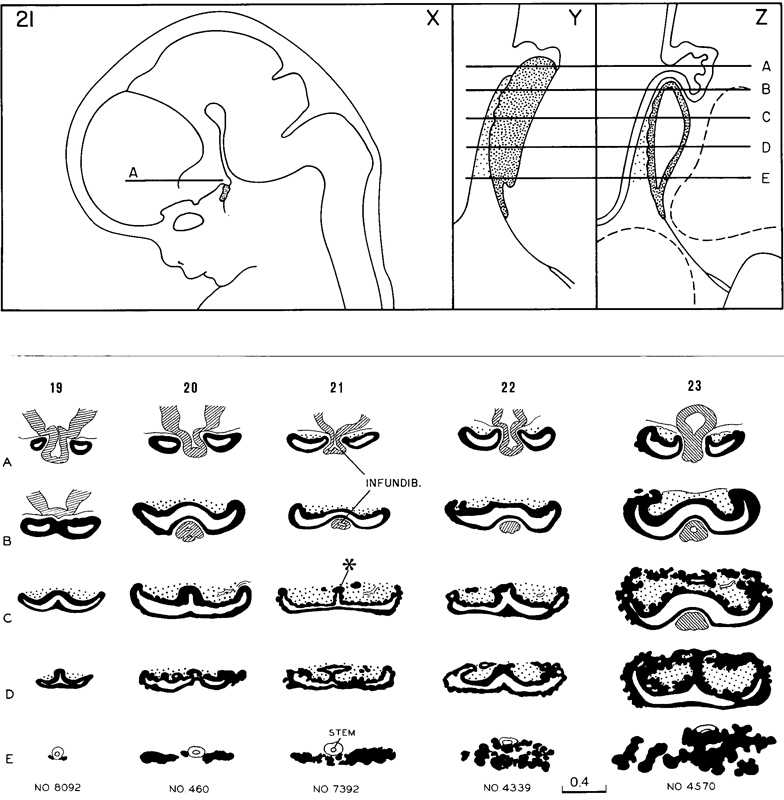
Fig. 19-8. The uppermost row shows (X) a general view of the brain at stage 21 (Aindicates the neurohypophysis), (Y) a left lateral view of the hypophysis cerebri, and (Z) a median section. The transverse lines A–Ein Y and Z correspond approximately to the sections shown in the middle vertical row marked 21.
The lower part of the figure consists of semidiagrammatic drawings of transverse sections of the hypophysis at stages 19- 23. One embryo was chosen for each stage, and drawings were made at five more or less evenly spaced levels of the hypophysis. The changing pattern of the various parts of the gland can be followed, stage by stage, by scanning the horizontal rows A–E. All these drawings are at the same scale.
Page 247
Fig. 19-9. Drawings from the sections of the vomeronasal organ at stages 19–23. All drawings are at the same scale.
Page 248
Fig. 19-10. Semidiagrammatic drawings of the submandibular gland, showing the growth of the ductal system into the mesodermal component at stages 19–23. All drawings (except the inset) are at the same scale.
Page 249 Page 250
Fig. 19-11. Semidiagrammatic drawings showing the origin and development of the tubules in the metanephros at stages 19–21. Practically all the embryos of stage 19 show the beginning formation of renal vesicles. Most of the specimens of stage 20 have S-shaped lumina in the vesicles, and spoon-shaped capsules have appeared in a few of the more advanced members of the group. The tubules in stage 21 show a wider range of development because new ones are being added peripherally; at the same time, those first established continue to differentiate.
Page 251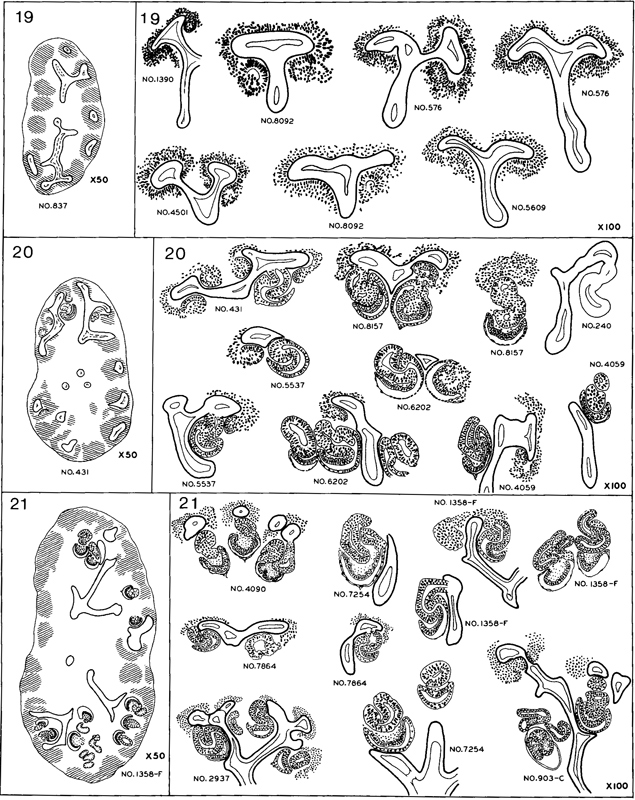
Fig. 19-12. A continuation of the previous illustration, showing the further development of the metanephros at stages 22 and 23. More large glomeruli have appeared, and short secretory tubules are present in nearly all specimens. By the end of the period, tubules of the fourth or fifth generation have formed. All drawings in this and in the previous figure are at the same scale.
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
