Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 5
Page 23Approximately 0.1-0.2 mm
Approximately 7-12 postovulatory days
Characteristic features: implanted but previllous; solid trophoblast in 5a; trophoblastic lacunae, cytotrophoblastic clumps, and primary umbilical vesicle in 5b; lacunar vascular circle and some mesoblastic crests in cytotrophoblastic clumps in 5c
Stage 5 comprises embryos that are implanted to a varying degree but are previllous, i.e., that do not yet show definite chorionic villi. Such embryos are believed to be 7-12 days old. The chorion varies from about 0.3 to 1 mm, and the embryonic disc measures approximately 0.1-0.2 mm in diameter. The significant dimensions of Carnegie specimens of stage 5 are listed in Table 5-1. The external and internal diameters of the chorion are listed as “chorion” and “chorionic cavity,” respectively. Additional features of stage 5 include the definite appearance of the amniotic cavity and the formation of extra-embryonic mesoblast. The appearances at stages 2 to 5 are shown in figure 5-1.
Implantation, which began in stage 4, is the characteristic feature of stage 5. It should be appreciated that both maternal and embryonic tissues are involved in the complex process of implantation: “in the normal process they are mutually supporting and neither can be regarded as chiefly responsible” (Boyd and Hamilton, 1970). An indication of a decidual reaction appears during stage 5 and, from this time onward, the term “decidua” (used by William Hunter) is commonly employed. The decidua, at least in the human, “is a tissue made up of endometrial connective tissue cells which have enlarged and become rounded or polyhedral due to the accumulation of glycogen or lipoids within their cytoplasm, and which occur either in pregnancy, pseudo-pregnancy or in artificially or pathologically stimulated deciduomata” (Mossman, 1937).
Successful implantation may depend on the ability of the embryo to produce an immunosuppressive factor (or factors) having a direct suppressive effect on the maternal immune response (Daya and Clark, 1986). Failure of implantation may result from rejection of the antigenic embryo by the maternal immune system. Heuser’s technique of opening the uterus laterally and searching for a young conceptus has been described on several occasions (e.g., by Heard, 1957, and by Hertig and Rock, 1973).
No correlation has been found between the side of the uterus on which the conceptus becomes implanted and the ovary from which the oocyte originated. Normal specimens, however, are more commonly found implanted on the posterior wall of the uterus, abnormal ones on the anterior wall (Hertig and Rock, 1949). Both walls are considered to be antimesometrial in comparison with a bicornuate uterus. Furthermore, “it is interesting to note that cases are known of a double discoid placenta in man very similar to that of the monkey. It seems entirely possible that in some cases the human blastocyst may attach both dorsally and ventrally and therefore fail to undergo complete interstitial implantation” (Mossman, 1937).
The trophoblast from stages 4 and 5 onward comprises two chief varieties, namely, cytotrophoblast and syncytiotrophoblast. That the latter is derived from the former had long been suspected and has been shown by organ culture and also indicated by electron microscopy (Enders, 1965).
An amniotic cavity is found by stage 5. If duplication of the embryo occurs after the differentiation of the amnion, the resulting monozygotic twins should be monochorial and monoamniotic (fig. 5-2). It has been estimated that the frequency of monoamniotic twins among monozygotic twins is about 4 percent (Bulmer, 1970). About once in every 400 monozygotic twin pregnancies, the duplication is incomplete and conjoined (“Siamese”) twins (e.g., the second specimen of Shaw, 1932) result.
The following description of stage 5 is based largely on the work of Hertig and Rock, in whose publications (1941, 1945a, 1949) much additional information (including descriptions of the ovaries, uterine tubes, and uterus) can be found. Based on the condition of the Page 24 trophoblast and its vascular relationships, stage 5 is subdivided into three groups: 5a, 5b, and 5c (Hertig, Rock, and Adams, 1956).
Table 5-1. Significant Dimensions (in mm) of Carnegie Specimens of Stage 5
| Stage | 5a | 5a | 5a | 5b | 5b | 5b | 5b | 5c | 5c | 5c | 5c | 5c |
| Serial No. | 8225 | 8020 | 8155 | 8215 | 8171 | 8004 | 9350 | 7699 | 7950 | 7700 | 8558 | 8330 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chorion | 0.33 x 0.306 x 0.12 | 0.45 x 0.30 x 0.125 | 0.306 x 0.210 x 0.15 | 0.525 x 0.498 x 0.207 | 0.422 x 0.404 x 0.256 | 0.582 x 0.45 x 0.31 | 0.599 x 0.58 x 0.36 | 1.026 x 0.713 x 0.515 | 0.75 x 0.45 | 0.948 x 0.835 x 0.54 | 0.96 x 0.52 | 0.85 x 0.65 |
| Chorionic cavity | 0.228 x 0.20 x 0.03 | 0.288 x 0.186 x 0.044 | 0.168 x 0.082 x 0.066 | 0.228 x 0.21 x 0.10 | 0.164 x 0.138 x 0.08 | 0312 x 0.185 x 0.12 | 0.3 x 0.1 x 0.1 | 0.48 x 0.336 x 0.276 | 0.40 x 0.26 | 0.55 x 0.498 x 0.425 | 0.58 x 0.36 | 0.46 x 0.40 |
| Trophoblast | 0.006 - 0.086 | 0.003 - 0.09 | 0.003 - 0.08 | 0.013 - 0.16 | 0.04 - 0.13 | 0.035 - 0.175 | 0.064 - 0.153 | 0.04 - 0.15 | 0.0125 - 0.185 | 0.02 - 0.28 | 0.10 - 0.24 | |
| Embryonic disc | 0.09 x 0.078 x 0.036 | 0.126 x 0.092 x 0.044 | 0.09 x 0.05 x 0.03 | 0.084 x 0.052 x 0.05 | 0.114 x 0.088 x 0.046 | 0.132 x 0.10 x 0.046 | 0.132 x 0.09 x 0.05 | 0.138 x 0.138 x 0.089 | 0.16 x 0.07 | 0.204 x 0.165 x 0.045 | 0.22 x 0.08 | 0.216 x 0.063 |
| Amniotic cavity | 0.06 x 0.054 x 0.006 | 0.025 x 0.024 x 0.003 | 0.048 x 0.032 x 0.02 | 0.05 x 0.048 x 0.022 | 0.04 x 0.036 x 0.024 | 0.078 x 0.066 x 0.012 | 0.108 x 0.099 x 0.024 | 0.02 x 0.014 | 0.174 x 0.14 x 0.0125 | 0.216 x 0.036 | 0.16 x 0.05 | |
| Primary umbilical vesicle | 0.08 x 0.023 | 0.043 x 0.06 | absent | 0.246 x 0.168 x 0.124 | 0.33 x 0.19 | 0.474 x 0.4l x 0.29 | 0.49 x 0.30 | 0.35 x 0.31 | ||||
| Reference | Hertig and Rock (1945b) | Hertig and Rock (1945a) | Hertig and Rock (1949) | Hertig and Rock (1945c) | Hertig and Rock (1949) | Hertig and Rock (1945a) | Heuser (1956) | Hertig and Rock (1941) | Hertig, Rock, and Adams (1956) | Hertig and Rock (1941) | Hertig, Rock, and Adams (1956) | Hertig, Rock, and Adams (1956) |
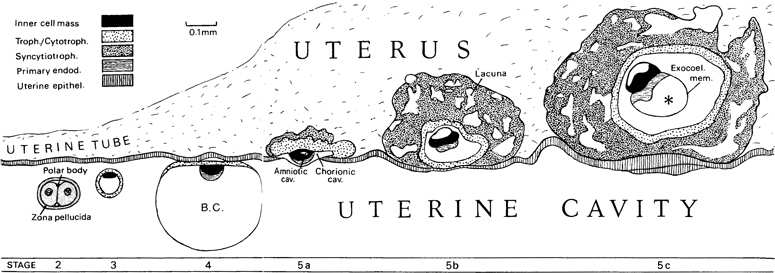
Fig. 5-1. Drawings of the embryo from stage 2 to stage 5c to show implantation. The drawings, which are all at the same scale of magnification, are based on human specimens, with the exception of stage 4, for which a macaque blastocyst was used. B.C., blastocystic cavity. Asterisk, primary umbilical vesicle.
Although a brief description of the trophoblast at each stage is provided in this account, the main emphasis is devoted to the embryo itself. This is justifiable inasmuch as comprehensive books on the human trophoblast (Hertig, 1968) and the human placenta (Boyd and Hamilton, 1970; Ramsey, 1975; Ramsey and Donner, 1980) have been published.
STAGE 5a
The characteristic feature of subdivision 5a is that the trophoblast is still solid, in the sense that definitive lacunae are not yet evident. Specimens of this stage are believed to be 7-8 days old. The chorion is less than 0.5 mm in its greatest diameter, and the embryonic disc is approximately 0.1 mm in diameter. Because of the collapse of the conceptus during implantation, the blastocystic cavity is flattened.
The rarity of specimens of stage 5a has been attributed to the circumstance that they are “impossible to discern in the fresh, and probably often unrecognizable even after fixation” (Hertig, 1968). Endometrium. The endometrial stroma is edematous (fig. 5-4). Two specimens (Nos. 8020 and 8225) show early, superficial implantation. The conceptus has eroded the surface epithelium of the uterus and has barely penetrated the underlying stroma (fig. 5-5). Apparently an attempt has been made by the maternal epithelium to repair the defect, and occasional mitotic figures are found. A portion of the conceptus, however, is still exposed to the uterine cavity. A third specimen (No. 8155) shows later, interstitial implantation. The conceptus is almost embedded within the endometrium, so that its abembryonic pole, which is barely exposed, is nearly flush with the epithelial lining of the uterine cavity (fig. 5-6).
Trophoblast. The trophoblast may encroach on the surrounding endometrial glands. At the abembryonic Page 26 pole, the wall of the conceptus is merely a thin layer of cells that resembles mesothelium. Because this region is not in contact with maternal tissue, it probably presents the structure of the wall of the blastocyst a it was at the time of implantation. Because of the collapse of the blastocyst during implantation, the mesothelioid layer is closely applied to the ventral surface of the embryonic disc (fig. 5-5).
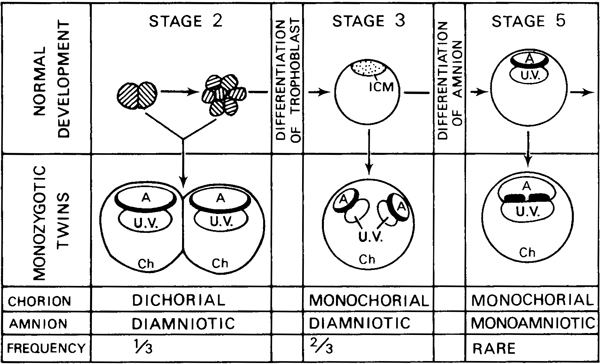
Fig. 5-2. Diagram to illustrate the presumed mode of development of monozygotic twins in the human. Based partly on Corner (1955). There exist “three critical stages at which the division of the embryo to form monozygotic twins may occur” (Bulmer, 1970). At stage 2, before the differentiation of the trophoblast, separation of the blastomeres would result in twins with separate choria and amnia. At stage 3 (and presumably at stage 4) before differentiation of the amnion, duplication of the inner cell mass would result in twins with a common chorion but separate amnia. At stage 5, duplication of the embryonic disc would result in twins with a common chorion and amnion. Deceptive fusion of the membranes may occur subsequently in certain instances but “the placenta and membranes, if subjected to skilled examination, including microscopic study of the chorionamniotic walls when necessary, will generally yield a correct impression of the type of twinning” (Corner, 1955; see also Allen and Turner, 1971). Partial instead of complete embryonic separation would result in conjoined twins of the various types classified by Wilder (1904).
As the mesothelioid layer is traced laterally, it becomes continuous first with indifferent trophoblastic cells, which, at the embryonic pole, become differentiated into cytotrophoblast and syncytiotrophoblast. Definitive trophoblast is found only in the area of endometrial contact, presumably under the influence of an endometrial factor. A ventrodorsal gradient of trophoblastic differentiation is noticeable. In other words, the most highly developed trophoblast tends to be found deeply (i.e., away from the uterine cavity).
The cytotrophoblast is located nearer the embryonic disc. The cells are large and polyhedral, and show distinct cell boundaries. Mitotic figures are moderately frequent.
The syncytiotrophoblast is described by Hertig (1968) as “invasive, ingestive, and digestive.” It presents a dark, homogeneous cytoplasm, and large, densely stained nuclei. No mitotic figures are seen. Near the maternal tissue, the syncytiotrophoblastic mass displays numerous small nuclei, which appear to be formed amitotically, although some may perhaps be derived from the endometrial stroma. The syncytial masses project into, and frequently partly surround (“eat their way into”), the uterine stroma, giving the surface of the trophoblast a lobulated appearance. In only rare instances are vacuoles found in the syncytiotrophoblast, and they contain no maternal blood (No. 8020). Macroscopically, no congestion or hemorrhage is visible Page 27 in the endometrium. Microscopically, the capillary plexus and sinusoids are moderately dilated but contain very few blood cells. It seems that the endometrium itself is adequate for the nourishment of the conceptus at this stage.
Fig. 5-3. Surface view of the implantation site of No. 8020, stage 5a, photographed under liquid. The dark ring indicates the chorionic cavity. The opaque area within the ring represents the embryonic mass; that outside of the ring represents the trophoblast. The mouths of the endometrial glands appear as dark spots.

Fig. 5-4. General view of the tissues at and near the implantation site (No. 8020, stage 5a). The endometrium is edematous. Section 6-5-9.

Fig. 5-5. Section through the middle of No. 8020, stage 5a. The amniotic cavity and the bilaminar embryonic disc can be seen. The transition from the thin abembryonic trophoblast to the thick, solid layer at the embryonic pole is evident. Large multinucleated masses of syncytiotrophoblast project into the endometrial stroma. A dilated endometrial gland is cut through at the left-hand side of the photomicrograph. Section 6-5-9.
Page 28It has been found (in Nos. 8020 and 8225) that “the course of several capillaries can be followed through the syncytiotrophoblast. The endothelial walls of the capillaries are intact to the point where each vessel enters and leaves the trophoblast, but between these points red blood cells can be seen to occupy a series of irregularly shaped spaces, which are in continuity with one another” (Harris and Ramsey, 1966). These spaces, however, “are unlike the well-rounded vacuoles occasionally observed in syncytiotrophoblast,” and they are “much smaller than the definitive lacunae” present within a couple of days. It is assumed that “the syncytium advances by a flowing movement that engulfs the blood vessels” in the capillary plexus of the adjacent stratum compactum (ibid.). Isolated endothelial cells in the lacunae may lend support to the supposition that capillaries have been engulfed (Dr. Ramsey, personal communication, 1972). In other words, the future lacunae, which are usually in continuity with maternal vessels (No. 8020) “are interpreted to be derived from engulfed maternal vessels” (Böving, 1981).
Trophoblast and endometrium (in No. 8020) are intimately related, and no cellular boundaries can be seen by light microscopy. It is probable that uterine epithelial cells have been phagocytized prior to autolysis, although it is possible that they are fused with the trophoblast (Enders, 1976). Indeed, it has been claimed that the appearances seen in the rabbit (fusion of a uterine “symplasma” with the syncytiotrophoblast) may apply also to No. 8020 in the human (Larsen, 1970). Implantation in the rhesus monkey has been studied by electron microscopy, including the spreading of trophoblast along the basal lamina of the uterine epithelium, the breaching of the basal lamina, and cytotrophoblastic proliferation (Enders, Hendrickx, and Schlafke, 1983).
Extra-embryonic mesoblast. The term mesoblast is preferred by Hertig and Rock (1941) to mesenchyme (“rather nonspecific”), primitive mesoderm (“one might unintentionally imply some connection with the embryonic mesoderm”), or magma reticulare (which “refers to the more mature characteristics of this tissue”).
The magma has also been considered as merely a “degenerative remnant of primary yolk sac endoderm” (Luckett, 1978).
The formation of mesoblast begins in stage 5a. The theory that the extra-embryonic mesoblast develops in situ by “delamination” (i.e., without cellular migration) from the cytotrophoblast (Hertig and Rock, 1945a and 1949) remained current for many years, although other possible sources (embryonic disc, amniotic ectoderm, and endoderm) were also considered.
In his study of the Miller (5c) specimen, Streeter (1926) concluded that the primary mesoblast “must have either separated off from the inner cell mass during the formation of the segmentation cavity or have been derived from the trophoblast. Since it is, in reality, so largely concerned in the differentiation of the trophoblastic structures, the latter is the more probable explanation.” From his investigations of the same specimen and more particularly of macaque embryos, Hertig (1935) believed in the “simultaneous origin of angioblasts and primary mesoderm by a process of delamination of differentiation from the chorionic trophoblast....”
According to the alternative view, namely that extraembryonic mesoblast is not of trophoblastic origin, the trophoblast is considered to give rise to additional trophoblast only: i.e., cytotrophoblast, syncytiotrophoblast, and trophoblastic giant cells (Luckett, 1978).
Hill’s (1932) studies of the primate embryo led him to believe in “the existence in early embryos of the Pithecoid and man of a mesodermal proliferating area involving the postero-median margin of the shieldectoderm and the immediately adjoining portion of the amniotic ectoderm which contributes to, if it does not entirely form, the connecting stalk primordium. This proliferating area, it may be suggested, functionally replaces, if it does not actually represent, the hinder end of the primitive streak of the Tarsioid and the Lemuroid....” In pursuance of this idea, Florian (1933) concluded that, in the Werner (5c) embryo, an “area of fusion of the ectoderm of the caudal part of the embryonic shield with the primary mesoderm” was a site where “at least a part of the primary mesoderm originates,”
This theory of Hill and Florian has been supported by Luckett (1978) who believes that “the caudal margin of the epiblast is a precociously differentiated primitive Page 29 streak, which gives rise to the extraembryonic mesoderm of the chorion, chorionic villi, and body stalk.” The term caudal mesoblast-proliferating area will be retained here and, as in previous studies, identification of the primitive streak will await stage 6b, an interpretation that has long met with general agreement.

Fig. 5-6. Section approximately through the middle of No. 8155, stage 5a. The amniotic cavity (“trophoepiblastic cavity?“) and the bilaminar embryonic disc can be seen, although an amnion as such is not yet distinct. The thick, solid trophoblast at the embryonic pole is mainly syncytiotrophoblast. The endometrial stroma is edematous. Section 4-4-8.
Amnion. The cells of the inner cell mass that are adjacent to the mural trophoblast at stage 4 may already be those of the amniotic ectoderm. In stage 5a, the small space that appears within the inner cell mass, or in some instances seemingly between the mass and the trophoblast, represents the beginning of the amniotic cavity. Either the amnion itself or the amniotic cavity may be noticeable first.
In one instance (No. 8225), a single layer of flattened cells is found attached to the trophoblast although the amniotic cavity is scarcely present. In another case (No. 8155), by contrast, a prominent amniotic cavity (formed by the curved epiblast) is present although amniogenesis has not yet begun (fig. 5-6). In a third specimen (No. 8020), a small cavity is visible and amniogenesis is under way (fig. 5-5). The amniogenic cells, which are believed by Hertig and Rock (1945a) to be delaminating from the trophoblast dorsal to the embryonic disc, appear to be in the process of enclosing the amniotic cavity by fusing with the margin of the germ disc, In summary, the roof and lateral walls of the amniotic cavity are, in Hertig’s (1968) view, derived from the cytotrophoblast, and the cells are mesoblastic. The floor, however, is constituted by the epiblast.
In general terms, “two distinct methods of amnion formation are ordinarily considered: formation by folding and formation by cavitation, the latter being considered the more specialized” (Mossman, 1937). Both methods have been invoked by Luckett (1975) who, in an interpretation quite different from that of Hertig and Rock, has proposed that a primordial amniotic cavity appears within the embryonic mass (e.g., No. 8020), followed by opening of the roof to form a temporary tropho-epiblastic cavity (e.g., No. 8155). In stage 5b, it is maintained that the definitive amniotic cavity is formed by “upfolding of the margins of the epiblast” (e.g., No. 8215).
In a recent electron-microscopic study of the rhesus monkey (Enders, Schlafke, and Hendrickx, 1986) it was Page 30 concluded that the amniotic cavity appears as a result of a rearrangement of epiblastic cells (a “change in cell association occurring within epiblast”) whereby they are separated into amniotic ectoderm and epiblast proper.
The chief function of the amnion is not mechanical protection but rather the enclosing of “the embryonic body in a quantity of liquid sufficient to buoy it up and so allow it to develop symmetrically and freely in all directions” (Mossman, 1937).
Embryonic disc. The term “germ disc” was formerly employed for “the epithelial plate that is derived directly and exclusively from the blastomeric formative cells” (Heuser and Streeter, 1941). The plate may more conveniently be referred to as the epiblast. When the underlying primary endoderm is also included, the term “embryonic disc” (formerly “embryonic shield”) is used.
The embryonic disc (figs. 5-5 and 5-6) is bilaminar, composed of the epiblast and the primary endoderm. It is concavoconvex from dorsal to ventral.
The epiblast consists of variably sized, polyhedral cells which either show no precise pattern of arrangement (No. 8020) or are in the form of a pseudostratified columnar epithelium (No. 8155). One or more mitotic figures may be encountered.
The primary endoderm consists of a cap of small, darkly staining, vesiculated cells without any specific arrangement. Mitotic figures are not noticeable.
SPECIMENS OF STAGE 5a ALREADY DESCRIBED
Carnegie No. 8225. Described briefly by Hertig and Rock (194513). Hysterectomy (bicornuate uterus). Anterior wall of uterus. Chorion, 0.33 x 0.306 mm. Chorionic cavity, 0.228 x 0.2 mm. Embryonic disc, 0.09 x 0.078 mm. Perhaps more advanced than No. 8020 (Mazanec, 1959; Harris and Ramsey, 1966), but has also been interpreted as less advanced (Hertig, Rock, and Adams, 1956). Photomicrograph in Hertig, Rock, and Adams (1956, fig. 9). Presumed age, 7 days.
Carnegie No. 8020 (figs. 5-3 to 5-5). Described by Hertig and Rock (1945a). Hysterectomy. Posterior wall of uterus. Chorion, 0.45 x 0.3 mm. Chorionic cavity, 0.288 x 0.186 mm. Embryonic disc, 0.126 x 0.092 mm. New model of blood vessels at implantation site has been prepared (Harris and Ramsey, 1966). Presumed age, 7 days.
Fruhling, Ginglinger, and Gandar (1954) described briefly a specimen of about 8 days. Curettage. Early implantation. Few trophoblastic digitations. Beginning amniotic cavity. Most sections through embryonic disc lost.
Carnegie No. 8155 (fig. 14). Described by Hertig and Rock (1949). Hysterectomy. Anterior wall of uterus. Chorion, 0.306 x 0.210 mm. Chorionic cavity, 0.168 x 0.082 mm. Embryonic disc, 0.09 x 0.05 mm. “Tropho-epiblastic cavity” (Luckett, 1975).
STAGE 5b
Subdivision 5b is characterized by the appearance of definitive lacunae in the trophoblast (fig. 5-9). The lacunae communicate with endometrial vessels, and “this joining of maternal vessels to trophoblast is the essence of the uteroplacental circulation of the so called hemochorial type” (Hertig, 1968). It will be recalled that “the normal mammalian placenta is defined as an apposition or fusion of the fetal membranes to the uterine mucosa for physiological exchange” (Mossman, 1937).
Amniogenesis is well under way, and the primary umbilical vesicle is developed to a variable degree. Cytotrophoblastic clumps begin to project into the syncytiotrophoblast. The chorion attains approximately 0.6 mm in its greatest diameter and hence is readily visible to the naked eye. The space outlined by the internal surface of the chorion is slightly flattened but is undergoing its greatest diameter and hence is readily visible to the naked eye. The space outlined by the internal surface of the chorion is slightly flattened but is undergoing distension. The embryo is about 9 days old, and the embryonic disc is approximately 0.1 mm in diameter.
Endometrium. The endometrial stroma shows an early decidual (commonly called “predecidual”) reaction. The conceptus is imperfectly covered by the uterine epithelium. In one specimen (No. 8171), “moderate numbers of leucocytes in the predecidual stroma immediately surrounding” the specimen were noted (Hertig and Rock, 1949). These cells, because of their absence elsewhere, were interpreted as a physiological response to the conceptus. They were “mainly lymphocytes, with lesser numbers of macrophages and polymorphonuclear neutrophils.”
According to Krafka (1941), “the decidual reaction is generally defined as the appearance of certain large, clear, epithelioid, vesiculated cells, 40 to 50 μm in diameter, Page 31 ovoid or polyhedral in form, tightly compressed against one another (owing to imbibition of edema fluid or to storage of glycogen), having a conceded origin from the typical fusiform stroma cells primarily in the compacta.” The cells are not peculiar to the uterus (they may be found at tubal or ovarian implantation sites, for example), and hence the term “stroma” (better, “stromal”) reaction is preferred by Krafka.
Fig. 5-7. Surface view of the implantation site of No. 8004, stage 5b, photographed under liquid. The dark area in the middle represents the abembryonic wall of the specimen.

Fig. 5-8. General view of the tissues at and near the implantation site (No. 8004, stage 5b). The endometrium is “predecidual.” Section 11-4-4.
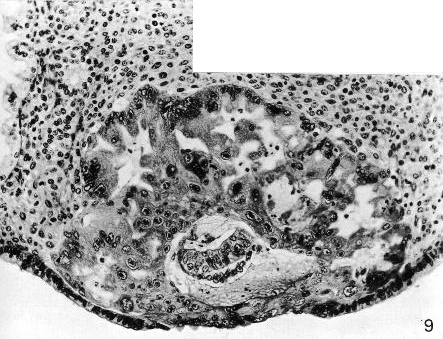
Fig. 5-9. Section through the middle of No. 8004, stage 5b. Some epithelial regeneration of the endometrium has taken place over the specimen. The large mass of syncytiotrophoblast shows intercommunicating lacunae. The chorionic cavity is surrounded by a thin layer of cytotrophoblast. The amniotic cavity and the bilaminar embryonic disc can be seen. Section 11-4-5.
Page 32Trophoblast. Although much of the trophoblast at the embryonic pole and at the equator is of the syncytial variety, an irregular inner rim of cytotrophoblast is present. In places, this rim has begun to form small, discrete masses (cytotrophoblastic clumps), which project into the syncytiotrophoblast and may be regarded as an indication of the future chorionic villi (fig. 6-3).
Various phases in the formation of lacunae within the syncytiotrophoblast are found (fig. 5-9). Most of these spaces have coalesced and, at several points, are in continuity with the dilated endometrial sinusoids. Few blood cells are seen in either the lacunae or the adjacent sinusoids, however, so that a uteroplacental circulation has scarcely been established. Rather, an ooze into the chorion occurs until chorionic villi appear at a later stage.
The origin of the lacunae has been investigated in the rabbit with the aid of electron microscopy. The study revealed “numerous vesicles in the cytoplasm of the syncytial trophoblast. These are the forerunners of larger cavities, approximately 30-40 μm in diameter,” which “by their confluence are later transformed into the lacunae of the definitive placenta” (Larsen, 1970). When the trophoblast penetrates the maternal vessels, the lacunae gain access to maternal blood (ibid.). According to Harris and Ramsey (1966) however, “during the initial stages of implantation in the human it appears that the syncytiotrophoblast engulfs the capillary plexus in the adjacent stratum compacturn. It is suggested that part of the plexus remains within the trophoblastic plate as a series of small spaces, which maintain continuity with the maternal circulation and subsequently enlarge to form the lacunae.”
Extra-embryonic mesoblast. The mesoblast continues to develop.
Chorion. The term chorion is commonly used “for the outer fetal membrane made up of trophoblast and somatic mesoderm” (Mossman, 1937), whether it be avascular (e.g., the “true chorion” of the pig) or vascular (e.g., the allanto-chorion of the human).
The mesoblastic lining of the trophoblast spreads during stages 5-7, and the combination of the two layers may now be termed chorion (Hamilton and Boyd, 1950). In stages 5b and 5c, however, the extent of the mesoblast is very slight, much or most of the extra-embryonic tissue is thought to be endoderm rather than mesoblast, and the chorionic cavity does not acquire a complete mesothelial lining until stage 6 or stage 7 (Luckett, 1978).
Amnion. The amniotic cavity, which is smaller than the umbilical vesicle, is almost closed by the amniogenie cells.
Embryonic disc. The bilaminar embryonic disc (fig. 5-9) resembles that seen at stage 5a.
The epiblast is a pseudostratified columnar epithelium in which the cytoplasm is beginning to become vacuolated ventrally.
The primary endoderm consists of either a single layer or a cap of cuboidal or polyhedral cells.
Umbilical vesicle. The primary umbilical vesicle is first seen in stage 5b and becomes limited by a layer that was formerly termed the exocoelomic membrane. This membrane is attached at the margin of the embryonic disc. The vesicle enclosed by the disc and the membrane “was first described and figured by Stieve (1931, 1936) in the Werner [5c] embryo” and, according to Davies (1944), he referred to this primary umbilical vesicle as the Dottersackanlage, although he made it clear that it is not the definitive umbilical vesicle as usually described.
The so-called exocoelomic membrane was described in the macaque as an “intrachorionic mesothelial membrane” that “must be regarded as a part of the primitive mesoblast” (Heuser, 1932a). In a later publication, however, the wall of the primary umbilical vesicle of the macaque was stated either to be derived from endodermal cells “which spread to line the chorion,” or to “arise by delamination from the trophoblast” (Heuser and Streeter, 1941). Elsewhere in the same article, the exocoelomic membrane is said to be derived either by delamination from the endoderm (misprinted as “endothelium”) or “by creeping in from the sides as a spreading membrane” of mesoblast.
In the human, the membrane and the embryonic Page 33 disc together enclose the primary umbilical vesicle. The suggested origin of the membrane in situ by delamination from the adjacent cytotrophoblast (Hertig and Rock, 1945a, 1949) and a similar mode of origin for scattered mesoblastic cells between the trophoblast and the membrane (Hertig and Rock, 1949) have been disputed. It has been reported that the primary umbilical vesicle “develops by the peripheral spread of extraembryonic endoderm,” which forms an epithelial meshwork within the blastocystic cavity (Luckett, 1978). Thus the so-called exocoelomic membrane is considered to be merely the wall of the primary umbilical vesicle, and the surrounding meshwork is thought to be extra-embryonic endoderm rather than mesoblast.
SPECIMENS OF STAGE 5b ALREADY DESCRIBED
Carnegie No. 8171. Described by Hertig and Rock (1949). Hysterectomy. Posterior wall of uterus. Abnormal leucocytic infiltration of endometrium. Chorion, 0.422 x 0.404 mm. Chorionic cavity, 0.164 X 0.138 mm. Embryonic disc, 0.114 x 0.088 mm. A cellular remnant within the umbilical vesicle, because it is probably derived from the endoderm, “may, in a sense, be regarded as an abnormal form of twin embryo” (ibid.). Presumed age, 9 days.
Carnegie No. 8215. Described briefly by Hertig and Rock (1945c). Hysterectomy. Posterior wall of uterus. Chorion, 0.525 X 0.498 mm. Chorionic cavity, 0.228 X 0.21 mm. Embryonic disc, 0.084 x 0.052 mm. Lacunae perhaps further developed than in No. 8171 (Mazanec, 1959), but specimen has been “considered to be slightly younger because the decidual reaction is not yet apparent” (Hertig, Rock, and Adams, 1956). Photomicrographs in Hertig, Rock, and Adams (1956, figs. 15 and 17). Presumed age, 9 days.
Carnegie No. 8004 (figs. 5-7 to 5-9). Described by Hertig and Rock (1945a). Hysterectomy. Posterior wall of uterus. Chorion, 0.582 x 0.45 mm. Chorionic cavity, 0.312 X 0.185 mm. Embryonic disc, 0.132 x 0.1 mm.
Carnegie No. 9350. Described briefly by Heuser (1956). Hysterectomy. At junction of posterior and anterior walls. Chorion, 0.59 x 0.58 mm. Chorionic cavity, 0.3 X 0.1 mm. Embryonic disc, 0.132 x 0.09 mm. Presumed age, 9 days.
STAGE 5c
Stage 5c is characterized by the intercommunication of the trophoblastic lacunae. The contained blood is sufficient to appear as a discontinuous red ring, so that identification of the conceptus is possible on careful gross examination of the endometrial surface prior to fixation (fig. 5-12). Embryos of stage 5c are believed to be 11-12 days old, the chorion measures about 0.75 to 1 mm in its greatest diameter, and the embryonic disc is approximately 0.15-0.2 mm in diameter. Some cytotrophoblastic clumps are beginning to acquire mesoblastic crests. The space outlined by the internal surface of the chorion now appears distended again.
In their proposals to subdivide horizon V, Hertig, Rock, and Adams (1956) distinguished 5b, “formation of trophoblastic lacunae with amniotic and exocoelomic cavities,” from 5c, “intercommunicating lacunae with beginning utero-lacunar circulation.” In 5b, “although the vast majority of the lacunar spaces have coalesced they contain relatively little maternal blood, and that mostly plasma, since few adjacent capillary sinusoids of the endometrial stroma communicate directly with the lacunar network as yet.” In 5c, “the lacunar spaces now intercommunicate and contain enough maternal blood” to enable specimens at this stage to be “easily identified on careful gross examination of the endometrial surface prior to fixation. Such lacunar blood appears as a discontinuous red circle about 1 mm in diameter” (fig. 5-12).
Because the quantitative changes between 5b and 5c may prove difficult to discern, and because a given specimen may not have been examined grossly prior to fixation, it was at first considered that the two substages should be combined here into one for reasons of practicality. Nevertheless, in order to avoid complicating further an already established system, and in order to continue the subdivision of an otherwise large and prolonged (4-day) group of embryos, it was decided to retain 5b and 5c. It is hoped that, perhaps by comparing the measurements and the appearances of given specimens in section with the photomicrographs of embryos already staged, it may be possible to assign new examples to their appropriate places. If not, they could be classified as merely stage 5.
The availability of human specimens, after reaching its lowest point at stage 4, increases greatly with the advent of stage 5c, and from now onward the account will be confined almost entirely to the human.
Endometrium. The endometrial stroma around the Page 34 conceptus again shows an early decidual (“predecidual”) reaction, and indeed decidua may be said to be present. The uterine epithelium continues its repair of the defect and, within the defect, a fibrinous, leucocytic, hemorrhagic coagulum may be present. Moreover, a “constant attempt on the part of maternal tissues to heal the endometrium persists until ... the conceptus is of six weeks’ developmental age” (Hertig, 1968).

Fig. 5-10. Surface view of the implantation site of No. 7700, stage 5c.
Fig. 5-11. Side view of the implantation site of No. 7700, stage 5c.
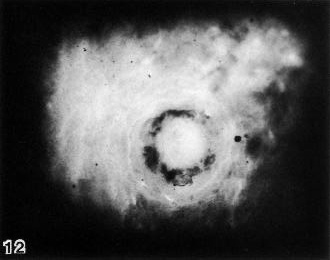
Fig. 5-12. View of the implantation site of No. 7700, stage 5c, as seen in the cleared celloidin block. The irregular, dark ring is caused by maternal blood within the trophoblastic lacunae, and is a characteristic feature of subdivision 5c, as it has been defined by Hertig, Rock, and Adams (1956).
The operculum, or closing plate, according to Kratka (1941), “is generally described as a simple organizing clot, including fibrin, fibrinoid, living and necrotic leucocytes, old and recent hemorrhage, and degenerating stroma.” That it is something more than a simple organizing clot, however, is indicated by the circumstance that “both the aperture and the operculum increase in size” (ibid.). The nomenclature of this region Page 35 (which includes Verschlusspfropf Schlusscoagulum, and Gewebspilz) has been clarified by Hamilton and Gladstone (1942), who defined the operculum deciduae (of Teacher, 1925) as the flattened or domeshaped head of the fungus- or mushroom-shaped structure, and the occlusion- or closing-plug as the part occupying and closing the aperture of entry.
Chorion. The cytotrophoblast, although it varies in thickness, is generally formed by a single layer of cuboidal cells. Masses of proliferating cytotrophoblast project into the syncytiotrophoblast. These cytotrophoblastic clumps (Davies, 1944), seen already at stage 5b, may be regarded as an indication of the future chorionic villi (fig. 6-3). In a few instances (in No. 7700) the beginning of a mesoblastic and angioblastic core has been detected. Such appearances may conveniently be termed mesoblastic crests (Krafka, 1941).
The syncytiotrophoblast forms about three-fourths of the total trophoblastic shell. It contains large, irregular, intercommunicating lacunae lined by a brush border and containing an increasing amount of maternal blood. The filling of the lacunae may result in a brilliant red circle (fig. 5-12), which enables the implantation site to be identified before fixation (Hertig, 1968). The presence of the lacunae gives the syncytiotrophoblast a spongy structure, and the lacunar system is primarily labyrinthine (Hamilton and Boyd, 1960). The implantation area presents intercommunicating capillaries, which, by several relatively large branches, communicate directly with the lacunae within the trophoblast. Also within the syncytiotrophoblast are found what appear to be phagocytosed maternal blood cells and possibly other tissues.
According to Park (1957), sex chromatin is absent up to 12 days, that is, during the first five stages. In one (abnormal) specimen (No. 8000) of 5c, an incidence of less than one percent was found in the trophoblast.
Extra-embryonic mesoblast. In addition to the continuing formation of mesoblast, angiogenesis is beginning at the abembryonic pole.
Amnion. The amniotic cavity seems to be nearly completely closed (fig. 5-14), although (in No. 7700) the appearances suggested to Hertig (1968, fig. 70) that amniogenic cells are still “being added to by the adjacent trophoblast.” The amniotic cavity is still smaller than the umbilical vesicle.
Embryonic disc. The bilaminar embryonic disc (fig. 5-14) resembles that seen at stage 5b. It is either basically circular on dorsal view, so that a longitudinal axis is not yet evident (No. 7699), or it is pyriform or ovoid, so that an apparent axis may be more or less envisioned (No. 7700 and No. 8139). It is possible also that a condensation of extra-embryonic mesoblast may serve to indicate the caudal end of the disc (Hertig, Rock, and Adams, 1956, fig. 38).
The epiblast is a pseudostratified columnar epithelium in which vacuoles are seen, mostly ventrally. Some mitotic figures may be observed.
The primary endoderm is sharply demarcated peripherally from the adjoining cells of the “exocoelomic membrane.” Mitotic figures, if found at all, are rare.
Umbilical vesicle. The so-called exocoelomic membrane (fig. 5-13), which was first figured by Stieve (1931) in the Werner embryo, is clearly formed, although it may be deficient in places.
Prechordal plate. It has been claimed that (chiefly in No. 8330) a thickening of the endoderm at the rostral end of the embryonic disc is the prechordal plate and is “the first clear evidence of a craniocaudal embryonic axis” (Luckett, 1978, fig. 19). The histological quality, however, does not permit definitive identification at this early stage.
SPECIMENS OF STAGE 5c ALREADY DESCRIBED
Davies-Harding. Described by Davies (1944). Hysterectomy. Anterior wall of uterus. Incomplete (almost one-half of embryonic disc missing). “No true villi.” Primary umbilical vesicle present. Extensive extra-embryonic meshwork. Slightly later stage of development than No. 8004 (Davies, 1944, Addendum), which belongs to 5b. “Possibly pathological” (Boyd and Hamilton, 1970). Chorion, 1.18 x 0.55 mm. Chorionic cavity, 0.409 x 0.238 mm. Embryonic disc, 0.117 mm. May be regarded as transitional between 5b and 5c. Presumed age, 9-10 days.
Carnegie No. 7699. Described by Hertig and Rock (1941). Hysterectomy. Posterior wall of uterus. Chorion, 1.026 x 0.713 mm. Chorionic cavity, 0.48 x 0.336 mm. Embryonic disc, 0.138 x 0.138 mm. New model of blood vessels at implantation site has been prepared (Harris and Ramsey, 1966). Presumed age, 11 days.
Carnegie No. 4900, Miller. Described by Streeter (1926). Curettage. Angiogenesis described by Hertig (1935). Incomplete (some sections missing). Primary umbilical vesicle present. New graphic reconstruction made by Streeter (1939a,b). Chorion, 0.9 mm. Chorionic cavity, 0.4 mm. Presumed Page 36 age, 10-11 days or perhaps even 12 days (Krafka,1941).
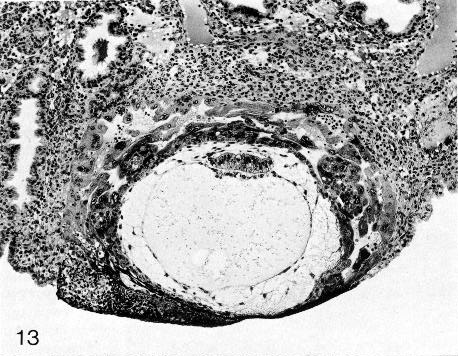
Fig. 5-13. Section through the middle of No. 7700, stage 5c. The space outlined by the internal surface of the chorion now appears distended again. However, a gradient of differentiating trophoblast from abembryonic to embryonic pole is still evident. Intercommunicating lacunae are visible in the syncytiotrophoblast. The primary umbilical vesicle, surrounded by a meshwork, can be seen. The endometrial stroma is edematous and deciduas is developing. Section 6-1-5.
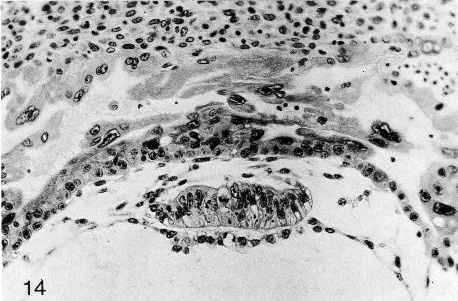
Fig. 5-14. The bilaminar embryonic disc of No. 7700, stage 5c. The amnion overlying the disc does not seem to be complete. The epiblast is a pseudostratified columnar epithelium. The surrounding cytotrophoblast is evident, and lacunae can be seen in the syncytiotrophoblast. Cytotrophoblastic clumps may be regarded as an indication of the future chorionic villi. Section 6-1-5.
Page 37Dible-West. Described by Dible and West (1941). Autopsy. Posterior wall of uterus. Incomplete. Chorionic cavity, 0.47 x 0.28 mm. Embryonic disc, 0.1 x 0.02 mm. Presumed age, 11-13 days.
Müller (1930) described an autopsy specimen. Only one section was near the embryonic disc.
Wilson (1954) found a specimen by endometrial biopsy. Chorion, 0.5 x 0.6 mm. Chorionic cavity, 0.47 x 0.24 mm. Between the amnion and the cytotrophoblast, “a small accumulation of fibroblastic cells probably represents the earliest stage of the Bauchstiel.” The endoderm is described as being “delaminated from the embryonic disc.” Presumed age, 11 days.
Carnegie No. 7950. Described briefly by Hertig and Rock (1944). Chorion, 0.75 x 0.45 mm. Chorionic cavity, 0.4 x 0.26 mm. Embryonic disc, 0.16 x 0.07 mm. Slightly more developed than No. 7699, although embryo is a little less differentiated. Photomicrographs in Hertig, Rock, and Adams (1956; figs. 27 and 35). Presumed age, 12 days.
Carnegie No. 7700 (figs. 18-22). Described by Hertig and Rock (1941). Hysterectomy. Posterior wall of uterus. Chorion, 0.948 x 0.835 mm. Chorionic cavity, 0.55 x 0.498 mm. Embryonic disc, 0.204 x 0.165 mm. Presumed age, 12 days.
Carnegie No. 8558. Measurements and photomicrographs in Hertig, Rock, and Adams (1956; figs. 30 and 37). Chorion, 0.96 x 0.52 mm. Chorionic cavity, 0.58 x 0.36 mm. Embryonic disc, 0.22 x 0.08 mm. Presumed age, 12 days.
Carnegie No. 8330. Measurements and photomicrographs in Hertig, Rock, and Adams (1956; figs. 30 and 37). Chorion, 0.85 x 0.65 mm. Chorionic cavity, 0.46 x 0.4 mm. Embryonic disc, 0.216 x 0.063 mm. Condensation of extra-embryonic mesoblast perhaps indicates “the beginning of axis formation” (ibid.). Presumed age, 12 days.
Kleinhans. Described by Grosser (1922). Autopsy. Only one section. Embryonic disc not seen. Chorion, 0.8 x 0.65 mm. Chorionic cavity, 0.35 x 0.15 mm.
Barnes. Described by Hamilton, Barnes, and Dodds (1943), and further by Hamilton and Boyd (1960). Hysterectomy. Posterior wall of uterus. Pathological edema in endometrium (Davies, 1944). Chorion, 0.931 x 0.77 x 0.737 mm. Primary umbilical vesicle and perhaps early differentiation of secondary vesicle (Luckett, 1978). Presumed age, 10-11 days or perhaps 12 days (Davies, 1944).
Werner (Prof. Werner Gerlach). Described by Stieve (1936), with graphic reconstruction by Florian. Autopsy. Chorion, 0.78 x 1.36 x 0.72 mm. Embryonic disc, 0.18 x 0.12 mm. Some large, round cells in the epiblast are mentioned as possible primordial germ cells. Primary umbilical vesicle and perhaps early differentiation of secondary vesicle (Luckett, 1978). Primordia (mesoblastic crests) of chorionic villi (Hertig and Rock, 1941). ‘Connecting stalk’ very indistinct. Rostrocaudal axis but no primitive streak; epiblast fused with extra-embryonic mesoblast caudally and perhaps is origin of latter (Florian, 1933 and 1945). Perhaps 12 days.
Knoth and Larsen (1972) studied an implantation site by electron microscopy. No villi, but “beginning” to form. Primary umbilical vesicle “not so easily seen.” Probably 11 days.
Dankmeijer and Wielenga (1968) described a specimen of stage 5. Curettage. Incomplete.
Carnegie No. 8139. Described by Marchetti (1945), who admitted that it is “not entirely normal.” Curettage. Chorion, 0.706 x 1.2 mm. Chorionic cavity, 0.635 x 0.582 mm. Embryonic disc, 0.126 x 0.048 x 0.116 mm. Embryo located centrally in chorionic cavity, which contains a meshwork. Embryonic disc, slightly ovoid (i.e., presents a longitudinal axis). Described originally as previllous, although “primitive villi” (stage 6) are mentioned by Boyd and Hamilton (1970): folds of undulating contour of cytotrophoblast are not yet “primitive unbranching villi, although they may be the primordial of them” (Marchetti, 1945; fig. 5). Primary umbilical vesicle present. May be regarded as transitional between stages 5 and 6. Presumed age, 13 days.
Bandler (1912) found a specimen embedded in tubal mucosa and which showed “as yet absolutely no suggestion of chorionic villi.” No details available.
Pommerenke (1958) described a specimen of stage 5. Curettage. Incomplete. Embryo not included.
Macafee. Described by Morton (1949). Curettage. Probably belongs to stage 5. Embryonic disc not found.
Scipiades (1938) described a specimen that belongs either to stage 5 or to stage 6, probably the former, “but its preservation is so poor that accurate conclusions are impossible” (Hertig and Rock, 1941). Curettage. Chorion, 1.498 x 0.49 mm. Chorionic cavity, 0.99 mm. Embryonic disc (only two sections available), 0.18 x 0.048 mm. Presumed age, 11-12 days.
Thomas and van Campenhout (1963) found a specimen that probably belongs to stage 5, although some trophoblastic thickenings of a villous character were said to be present.
Teacher-Bryce I. A pathological specimen of stage 5 described by Bryce (1924).
Sch. (Schönig). A pathological specimen of stage 5 described by von Möllendorff (1921a).
Keller and Keller (1954) found a pathological specimen embedded in the stroma of the ostium uteri.
Several pathological specimens of stage 5c are in the Carnegie Collection. Nos. 8370, 7770, 8299 (malpositioned embryonic disc, Hertig, 1968, fig. 132), 8329, 8000 (superficial implantation), and 7771 (no embryo) were measured and illustrated by Hertig, Rock, and Adams (1956).
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
