Developmental Stages in Human Embryos
Go to Stage: Intro 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Stage 9
Page 81Approximately 1.5–2.5 mm
Approximately 20 ± 1 postovulatory days
Characteristic feature: 1–3 pairs of somites
Now that the neural groove and the first somites are present, the “embryo proper” may be said to have been formed (van Oordt, 1921). The criteria used in distinguishing stages 5–9 are shown graphically in figure 9-1.
Stage 9 is defined by the number of somites present, namely 1–3 pairs. Although the degree of development is in general agreement with the number of somites, exceptions do occur.
At a certain period in vertebrate development, the number of pairs of somites that are clearly visible constitutes a simple and fairly accurate criterion for staging. Thus, in Ambystoma muculatum, stages 17–23 of Harrison show 1–6 somite pairs, respectively. In Gallus domesticus, a stage has been assigned to every third pair of somites that is added: stage 7, 1 pair; stage 8, 4 pairs; stage 9, 7 pairs; etc. In the human, greater spans of somitic pairs have been assigned to each stage. Thus, after the first 3 pairs have appeared at stage 9, stage 10 shows 4–12 pairs, stage 11 presents 13–20 pairs, and stage 12 possesses 21–29 pairs, after which time counting becomes more difficult and other criteria are emphasized.
In Streeter’s (1942) scheme, horizon IX was characterized by “neural folds, elongated notochord.” The neural folds, however, can appear during stage 8 (O’Rahilly and Gardner, 1971; O’Rahilly and Müller, 1981). Moreover, now that horizon X (“early somites present,” Streeter, 1942) has been limited specifically to “4 to 12 somites” (Heuser and Corner, 1957), it follows that the first 3 pairs of somites must appear earlier, namely, during stage 9.
Stages 1–3 are sometimes referred to as pre-implantation stages, stage 5 as previllous, stages 6–8 as presomitic, and stage 9 to approximately stage 13 as somitic stages.
Embryos of stage 9 are very rare. Fortunately two have been described in considerable detail (figs. 9-2 and 9-5). A great need exists, however, for further thorough accounts of specimens of excellent quality.
A detailed investigation of this stage was undertaken by Müller and O’Rahilly (1983), who provided graphic reconstructions.
SIZE AND AGE
Embryos of stage 9 vary from approximately 1.5–3 mm in length, and are believed to be about 20 days in age.
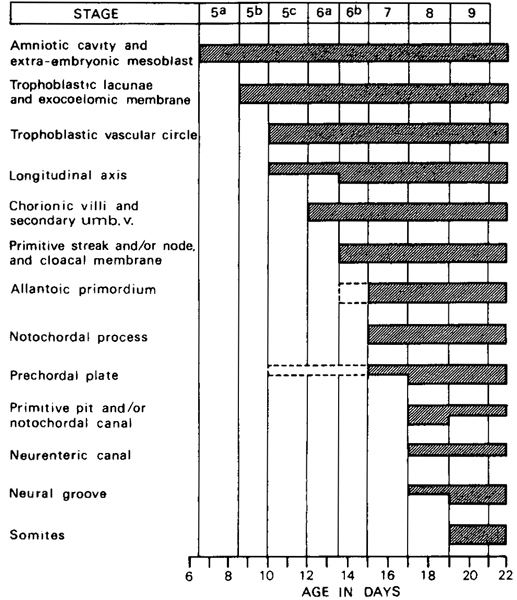
Fig. 9-1. Summary of the criteria used in distinguishing stages 5–9. The shaded bars indicate the stages at which a given feature is found. The following are not used: appearance of amniotic cavity, appearance of umbilical vesicle, branching of chorionic villi, cloacal membrane, allantoic diverticulum, prechordal plate, neural groove and neural folds.
Page 82EXTERNAL FORM
As seen from the dorsal aspect, the embryo is frequently described as shaped like the sole of a shoe (fig. 9-3).
Many, perhaps most, embryos of stages 9 and 10 display a dorsal concavity (“lordosis”) which has been subject to considerable discussion (fig. 9-4). Although abrupt bends and kinks are artifacts, “anything from a gentle convexity to a moderate dorsal concavity must be considered normal” (Heuser and Corner, 1957). An excellent example can be seen in figure 9-8. At stage 9, the rostral and caudal ends of the embryo begin to be elevated “above” (dorsal to) the level of the umbilical vesicle. It should be kept in mind, however, that a dorsal concavity is augmented by the collapse of the umbilical vesicle; indeed, wrinkling of the vesicle and dorsal flexure increase pari passu during dehydration (Bartelmez and Evans, 1926).
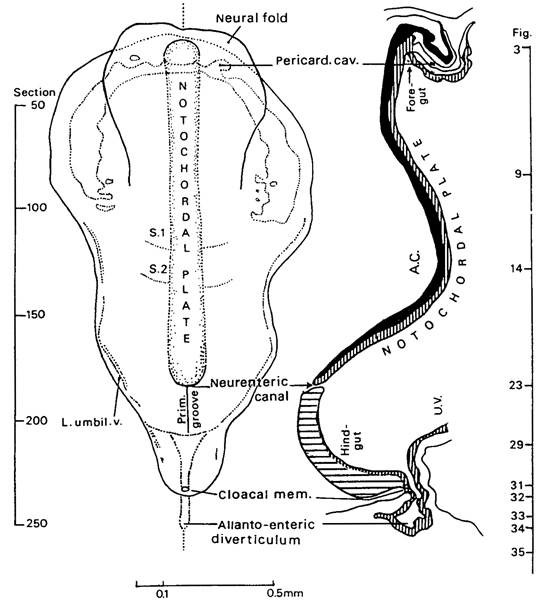
Fig. 9-2. Dorsal view and median reconstruction of embryo Da 1 (No. 5982), in alignment. The dorsal view, which is based on an illustration published by Ludwig (1928, fig. 2), shows the neural folds, pericardial cavity, notochordal plate, and somites. The median reconstruction is based on the same author (fig. 1). The foregut and hindgut are beginning to form, and the neurenteric canal and the allanto-enteric diverticulum are evident. The figure references are to the sections reproduced by Ludwig among an extensive series of photomicrographs.
Although an indication of a head fold may perhaps be detectable in a few embryos of stage 8, the caudal Page 83 fold does not appear until during stage 9 (in embryo Da 1). With the continuing elevation of the neural folds, lateral limiting sulci appear at first rostrally and then in the caudal part of the embryo (Da 1).
Studies of the chick embryo have led to the conclusion that “detachment of the head from the blastoderm is brought about to a lesser degree by independent head-fold formation, and to a greater degree by an influence of the fast-growing brain. Presence of detached foregut is a necessary prerequisite for head detachment” (Gruenwald, 1941a). Moreover, “we must abandon the conception of a simple folding of the blastoderm as the cause of detachment” not only for the rostral but also for the caudal end of the body and the corresponding gut. However, the process at the caudal end is “entirely different from that found in the head region, Here, too, the detachment is most probably due to growth of the body beyond its attachment to the blastoderm” (ibid.).
HISTOLOGICAL FEATURES
Amnion. An amniotic duct may be present (No. 1878).
Embryonic disc. Differentiation has now proceeded to the point where the various systems of the body can be discussed separately.
Primitive streak. The primitive streak extends from the cloacal membrane to the neurenteric canal. Rarely can its entire extent be appreciated in dorsal view (H3); usually it appears foreshortened (No. 1878) and may also be curved (Da 1). A primitive groove may be found but a distinct node may not always be readily distinguishable. When the obliquity and curvature of the streak are taken into account, the primitive streak occupies from one-third (Da 1; H3) to one-quarter (No. 5080; No. 1878) of the length of the embryo. This fraction becomes further reduced during stages 10 and 11 (Bartelmez and Evans, 1926).
Mesoderm. The mesoderm, as the mesoblast may now be termed, is arranged on each side as (1) a longitudinal, paraxial band (nicely shown in Ludwig’s reconstruction, plate 2, figs. 3 and 4), and (2) a lateral plate. The paraxial mesoderm is beginning to become segmented in the junctional region with the spinal cord, i.e., RhD (Müller and O’Rahilly, 1983; O’Rahilly and Müller, 1984b).
Although no nephric structures are found until stage 10, the general region of the intermediate mesoderm can be made out in stage 9 between the paraxial mesoderm and the lateral plate. (It is particularly clear, for example, on the right side of sections 115–117 of embryo Da 1.)
Somites. Somites are first visible at stage 9, and the 1-3 pairs present are presumed to be occipital. The first pair appears immediately caudal to the level of the midpoint of the notochordal plate (No. 5080). The somites may differ in number on the two sides of the body (No. 1878). Cavities (the so-called myocoeles) may be detectable in the somites (fig. 9-11).
Coelom. The appearance of the coelomic cavity has the effect of splitting the lateral plate into somatopleuric and splanchnopleuric layers.
The pericardial cavity (figs. 9-2, 9-7, 9-9, and 9-10) is a constant finding. It appears as a horseshoe-shaped space, together with associated vesicles, within the mesoderm of the rostral half of the embryo (No. 5080, Davis, 1927, figs. 2 and 3; Da 1, Ludwig, 1928, fig. 2). The limbs of the horseshoe begin blindly on each side at the level of the first somite. Here the cavities closely approach the extra-embryonic coelom although the intra-embryonic coelom remains closed throughout. At first the right and left limbs do not intercommunicate (No. 5080) but soon do so (Da 1; No. 1878). The small, discrete vesicles associated with the limbs of the horseshoe are coelomic spaces that have not as yet joined the larger cavities. They suggest the mode of formation of the coelomic cavity, namely the fusion of discrete vesicles. Some indications of coelomic formation caudally may be detected (Ludwig, 1928) and distinct bilateral cavities are present in one specimen (fig. 9-13).
Notochordal plate. The notochordal plate (about 0.66 mm in length in Da 1) is intercalated into the endoderm (fig. 9-2). It extends rostrally almost as far as the oropharyngeal membrane, and, caudally, it blends with the primitive node. Extracellular granules in the prechordal plate, notochordal process, primitive streak, and neural plate have been noted in presomitic and early somitic embryos (Allan, 1963).
Neurenteric canal. The neurenteric canal (fig. 9-2) may be completely patent (Da 1; H3; see Wilson, 1914, fig. 7) only partly patent (No. 5080), or completely closed (No. 1878). The neurenteric canal appears first during stage 8 (e.g., in Dy, R.S., M’Intyre, Gläveke, Vuill.) Page 84 and is found in some embryos of stages 9 and 10.

Fig. 9-3. Left lateral and dorsal views of No. 1878, as depicted by James F. Didusch in 1919. Cf. figures 9-4 and 9-5. Approximately one-half of the longitudinal extent of the neural groove represents the future brain.
Persistence of the neurenteric canal (Dodds, 1941), associated with duplication of the notochord (Saunders, 1943), may be important in the production of combined anterior and posterior rhachischisis. Frequently a patent connection between gut and spinal cord is present (Gruber, 1926; Dénes et al., 1967).
Prechordal plate. The prechordal plate consists of several layers of cells that resemble those of the neural plate. Rostrally, the prechordal plate usually separates from the neural plate and becomes continuous laterally with the cardiac mesoderm. The fusion of the rostral end of the notochordal plate with the mesoderm in Da 1 was thought by Hill and Florian (1931b) to be Page 85 the prechordal plate. It was believed to be identifiable in embryo Gv also.
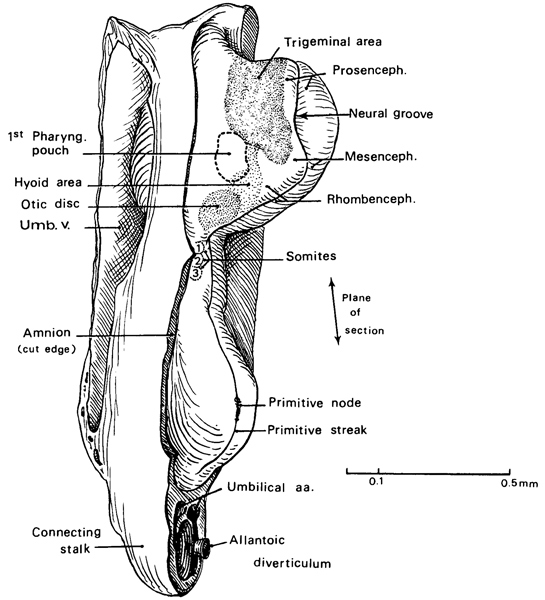
Fig. 9-4. Left lateral view of No. 1878. Based largely on a geometric projection of a reconstruction reproduced by Bartelmez and Evans (1926, plate 3, fig. 3). The ectodermal areas of the cephalic region are indicated by stippling. The mesencephalic flexure of the brain is evident.
The location of the prechordal plate in the human appears to correspond to “the axial mesoderm between the first pair of somitomeres” in the mouse (Meier and Tam, 1982). These authors found seven “segmental units” or “somitomeres” (derived from the primitive streak) in the paraxial region rostral to the first somite, so that “the cranial axis of the mouse embryo is initially organized into segments like the rest of the body.” Furthermore, their morphogenesis “is coordinate with neurulation.”
The prechordal plate, which is in the longitudinal axis of the embryo at stage 8 (O’Rahilly and Müller, 1981, fig. 4), gradually becomes rotated in association with the head fold, so that it comes to lie at a right angle. The cardiogenic plate, which merges with the prechordal plate material, is also undergoing reversal.
Page 86Umbilical vesicle (fig. 9-9). Blood islands are numerous and a vitelline plexus is visible. A small diverticulum of the umbilical vesicle has been described (No. 1878).
Cloacal membrane. Even now, this union of ectoderm and endoderm “is in a way hardly a membrane” (Ingalls, 1920).
Allantoic diverticulum. Either an allanto-enteric (Da 1) or an allantoic (No. 1878) diverticulum may be present. It runs between the umbilical arteries (fig. 9-4).
In the chick, rotation at the caudal end of the embryo alters the allantois in such a way that it becomes partly unfolded (Gruenwald, 1941a). The allantois then becomes a shallow diverticulum of the hindgut (ibid., fig. 6) although “it is highly probable... that the proximal part of the allantoic pocket contributes to the formation of the floor of the hindgut.”
Connecting stalk. The vascular channels are well developed and occupy most of the stalk, which is quite thick. The right and left umbilical arteries are readily distinguishable, one on each side of the allantoic diverticulum. The future umbilical veins are represented by a vascular plexus which communicates with the arteries. Blood islands have been identified in the connecting stalk as well as over the umbilical vesicle.
CARDIOVASCULAR SYSTEM
Blood vascular system. Blood vessels are arising in several separate regions (Ingalls, 1920): the chorion, the connecting stalk, the umbilical vesicle, and the embryo proper, together with its amnion. The connections between these vessels are secondary, and they are established at various times and in a number of different places.
Within the body of the embryo, the two omphalomesenteric veins are distinguishable. Each enters the corresponding horn of the sinus venosus. Also present within the body are the first pair of aortic arches, the beginning of the internal carotid arteries, and, at least in an interrupted course, the two dorsal aortae (Ingalls, 1920). A closed circulation, however, is not yet present. The aortae constitute medial vascular tracts, whereas lateral tracts consist of the vitelline veins and the cardiac rudiments (Ludwig, 1928, plate 3, fig. 6). It seems that the connection of the vitelline plexus with the aorta caudally antedates the connection with the heart rostrally (Ingalls, 1920). In No. 1878 the sole union of intra- and extra-embryonic vessels is provided by the aorta, which communicates (at least unilaterally) with the vitelline plexus. Either no blood cells (Da 1) or a few cells in the aorta (No. 1878) are found within the body of the embryo.
Heart
From his study of No. 5080, Davis (1927) concluded that, whereas the possibility of an extra-embryonic origin for the cardiac primordium could not definitely be excluded, “it can be stated with certainty that no connection exists between the well-differentiated angioblastic tissue of the yolk-sac and that of the heart.” In brief, “the evidence strongly leans toward an intra-embryonic origin, that is, from the cardiogenic plate.” The cardiogenic plate (of Mollier) is the ventral (splanchnic) wall of the pericardial cavity, and from it the myocardium is believed to be derived.
The endocardium (fig. 9-7) is represented at first by a network of mesenchymal cells between the cardiogenic plate and the endoderm. This endocardial plexus, which is at first (Da 1) mostly solid and paired, soon becomes divisible into three parts: atrial, ventricular, and conal (bulbar). The cellular cords that form the plexus become canalized, and their cavities become confluent (Orts Llorca, Jiménez Collado, and Ruano Gil, 1960).
A heart may be said to be present in Nos. 1878 and 7650 (Müller and O’Rahilly, 1983, fig. 7). The development of the cardiac region of one or both of these embryos has been discussed by Davis (1927) de Vries and Saunders (1962), and de Vries (1981). The endocardium at stage 9 may be in the plexiform phase of Davis (1927), as in embryo Gv (Orts Llorca et al., 1960; Jiménez Collado and Ruano Gil, 1963). In Nos. 1878 and 7650, however, the heart would appear already to have entered the paired tubular phase (despite a statement to the contrary by Davis). Intra-embryonic blood vessels are most advanced in these same two embryos.
The conoventricular region (see O’Rahilly, 1971, for terminology) occupies the median plane in No. 1878, in which two ventricular roots unite with each other. Rostrally, the cardiac plexus gives way to the first pair of aortic arches, which in turn lead to the dorsal aortae. The atrial components remain bilateral until a subsequent Page 87 stage.

Fig. 9-5. Dorsal view and median reconstruction of No. 1878, in alignment. The dorsal view, which is based on an illustration by James F. Didusch (see fig. 9-3), shows the neural folds and groove, the otic discs, and the somites. Medial to the otic discs, areas that possibly represent the trigeminal ganglia are indicated without a label. The median reconstruction is based on an illustration by James F. Didusch (Ingalls, 1920, plate 2) and on a drawing reproduced by Bartelmez and Evans (1926, plate 5, fig. 9). Various features are shown: pericardial cavity, oropharyngeal membrane (O.P.M.), developing pharynx (Phar.), rostral intestinal portal, and allantoic diverticulum.
With regard to the external appearance of the heart, three sulci have appeared in No. 1878: (1) atrioventricular, (2) interventricular (bulboventricular), and (3) conotruncal (interbulbar; infundibulotruncal). It should be kept in mind, however, that the cardiac region of this embryo appears to be distorted.
Other features that have been identified by stage 9 include the “cardiac jelly” (Davis, 1927), the mesocardium, and the septum transversum (Ingalls, 1920).
DIGESTIVE SYSTEM
The foregut develops during stage 9 (or possibly but doubtfully at the end of stage 8) as a recess of the umbilical vesicle. The midgut and hindgut, however, are either still combined (No. 1878 and No. 5080) or else (fig. 9-2) the hindgut is making its appearance as a separate recess (Da 1). In the former case, the cloacal membrane is still in the roof of the gut and, immediately caudal to it, the allantoic diverticulum arises (fig. 9-5).
Page 88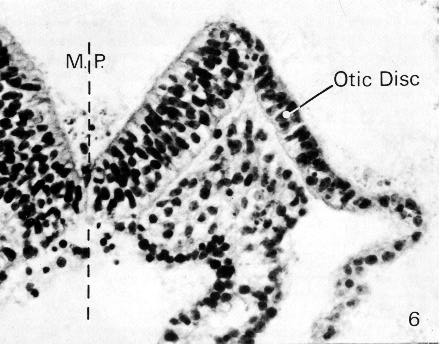
Fig. 9-6. The neural groove and one of the neural folds of No. 1878 in the region of the rhombencephalon. The otic disc is found on the lateral aspect of the neural fold. The basement membrane is visible. The interrupted line (M. P.) indicates the median plane. Section 12-5-7.
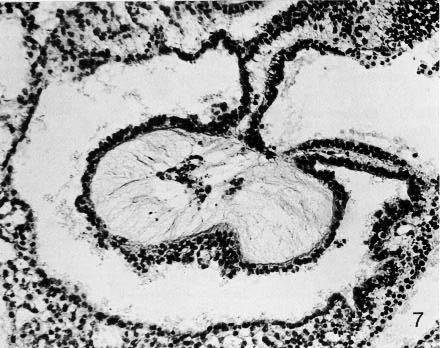
Fig. 9-7. Oblique section through the heart of No. 1878. The foregut appears near the upper right-hand corner of the photomicrograph. The U-shaped space is the pericardial cavity. The myocardial mantle, the “cardiac jelly,” and the endocardial plexus are evident centrally. Section 12-3-5.
Page 89In brief, although a rostral intestinal portal is present at this stage, a caudal portal may or may not be. In an excellent specimen that should be published in extenso (Prague No. 2008; 3 pairs of somites; embryonic disc, 1.73 mm), which one of the present writers examined through the kindness of Dr. J. E. Jirásek, the caudal fold, the hindgut, the caudal intestinal portal, and caudal coelomic cavities are all well marked (figs. 9-8 to 9-14).
From a study of abnormal chick embryos it has been concluded that, although “elevation of the head and proper development of the head fold largely depend on the condition of other structures of the head region,” the foregut “develops normally in the absence of the head fold as well as in cases in which the nearby anterior [rostral] end of the neural primordium is defective by malformation or experiment” (Gruenwald, 1941a). In other words, the foregut “depends very little upon the condition of the surrounding structures and develops normally whenever there is no mechanical obstacle” (ibid.).
The foregut is intimately related dorsally to the floor of the neural groove. At first a shallow pocket (of 0.17 mm in Da 1), the recess soon attains a length of 0.5 mm (in No. 1878). Caudally, the foregut appears triangular or even T-shaped on cross section, and it presents internally a trough in its floor as well as a corresponding ventral keel externally. The keel is closely related to the developing heart, and the trough indicates the general site of the future respiratory groove. In some embryos (Da 1 and Prague No. 2008), however, the keel is scarcely developed and the foregut is oval or reniform (fig. 9-9) rather than triangular in cross section. The “earliest trace” of the first pharyngeal pouches (fig. 9-4) and perhaps an “early indication” of the first pharyngeal cleft have been detected in one specimen (Ingalls, 1920).
It is now nearly half a century since, in a discussion of “fishy nomenclature,” it was proposed that the word “branchial” be dropped from mammalian embryology (Frazer, 1923). The visceral pouches of embryonic reptiles, birds, and mammals “bear little resemblance to the gill-slits of the adult fish” but rather “resemble the visceral pouches which appear in the embryonic stages of fish” (de Beer, 1958). Indeed, “all that can be said is that the fish preserves its visceral pouches and elaborates them into its gill-slits, while reptiles, birds, and mammals do not preserve them as such but convert them into other structures” (ibid.).
The oropharyngeal membrane is generally absent (e.g., in Da 1) but is quite well defined in one specimen (No. 1878) where it attains a width of about 0.05 mm (fig. 9-5). The stomodeum is beginning to form.
Although a pit in the ventral wall of the foregut has been claimed to represent the beginning of the thyroid gland (Wilson, 1914; Ingalls, 1920), it is likely that the thyroid primordium does not make its appearance until the following stage. Similarly, an indication of the liver is not found before stage 10.
In the chick embryo, the hindgut has been described as initially a hollowing out of the ventral portion of the “trunk-tail-node” and its “formation is not the result of a folding” of the blastoderm (Gruenwald, 1941a).

Fig. 9-8. Left lateral view of Prague embryo No. 2008, by courtesy of Dr. J. E. Jirásek. The gentle curvature of the body and the absence of kinking suggest the normal appearance to be expected at this stage. The umbilical vesicle is seen in the left-hand half of the photograph.
NERVOUS SYSTEM
Stage 9 heralds the onset of that developmental phase (largely stage 10) during which the neural folds dominate Page 90 the external picture. The neural groove, which appeared during stage 8, is now quite deep although it is still open throughout its entire extent (fig. 9-3). About one-half of the longitudinal extent of the groove represents the future brain. The area of the forebrain is conspicuous, and its neural folds are separated rostrally from each other by the terminal notch, which leads to the oropharyngeal membrane.
In the more advanced specimens of stage 9, the neural axis, at the caudal end of the forebrain, changes its direction through an angle of about 115 degrees (in No. 1878). This alteration of axis constitutes the mesencephalic (or cranial) flexure, which, in this and subsequent stages, occurs at the midbrain (fig. 9-4). The flexure is probably the result of the more rapid growth of the dorsal, as compared with the ventral, lamina of the midbrain (Bartelmez and Evans, 1926).
A distinct isthmus rhombencephali separates the midbrain from the hindbrain. The latter comprises four rhombomeres (proneuromeres of Bergquist and Källén).
The interval between the summits of the neural folds is narrowest in the junctional region between hindbrain and future spinal cord, and it is here that closure of the neural groove will first take place during stage 10.
It is frequently not appreciated that the three major divisions of the brain appear before any portion of the neural tube is present as such. The correct interpretation was clearly implied in an important paper by Bartelmez (1923), as stressed by Streeter (1927b), who discussed the myth of the brain vesicles in mammals and pointed out that “the brain begins to build its definitive parts before the closure of the neural tube.”
The division of the brain elaborated by Bartelmez (1923) is based on identification of (1) “the midbrain which is located by the cranial flexure,” and (2) the otic segment of the hindbrain, which is in close relation to the otic plate. “The hindbrain is the dominant feature of the brain in early stages. It is subdivided into three segments (of which the middle is the otic): RhA, RhB, and RhC.” However, in the opinion of the present writers, a fourth segment (RhD) should be included as part of the hindbrain because, being related to the rostralmost (i.e., occipital) somites, it must represent the hypoglossal region of the hindbrain (Müller and O’Rahilly, 1983, fig. 1).
Neural Crest
The head ectoderm is undergoing differentiation such that several areas (fig. 9-4) have been mapped out in one specimen (Bartelmez and Evans, 1926, plate 3, fig. 3): the otic disc (already mentioned), the trigeminal nerve area, the ectodermal area that will later cover the hyoid (second pharyngeal) arch, and the site of the first pharyngeal membrane (overlying the first pharyngeal pouch).
Although the neurosomatic junction is unclear at this stage, mitotic figures are more numerous at the presumed junction. This is especially marked in the mesencephalic region and to a lesser extent in the rhombencephalic area. These dividing cells are believed to be neural crest. Such areas are visible in at least two embryos (Vant and No. 7650) and represent probably the rostral crest and the facial crest. These two embryos are the earliest examples of the initial formation of neural crest, which is generally associated more with stage 10. Unfortunately the plane of section of No. 1878 is unsuitable for plotting the neural crest, which is probably present. A cellular collection near the otic disc has been claimed to be perhaps the primordium of the trigeminal ganglion (Ingalls, 1920).
Eye
Shaner (1945) believed that the “blunted tips” of the neural folds (his fig. 3) are the optic primordia. Although this is not impossible (see O’Rahilly, 1966, for discussion), it is generally maintained that the optic primordia appear first during stage 10.
Ear
The otic disc (or plate) makes its appearance during stage 9 (fig. 9-6). At first ill defined and merely suggested (Ludwig, 1928, fig. 9) it is soon (O'Rahilly, 1963) a better marked ectodermal thickening (in No. 1878; fig. 9-6) approximately opposite the middle of the rhombencephalic fold. The otic disc probably involves more ectoderm than is later incorporated into the otic vesicle (Bartelmez and Evans, 1926).
Page 91Figures 9-9 to 9-14 are sections through Prague embryo No. 2008, by courtesy of Dr. J. E. Jirásek.
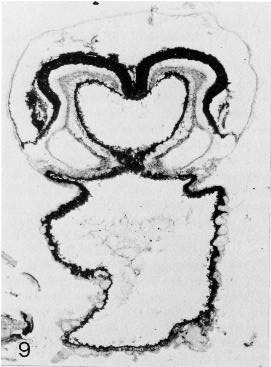
Fig. 9-9. The amniotic cavity, neural groove, foregut, and umbilical vesicle are evident. The foregut, which appears reniform, has been sectioned immediately rostral to the rostral intestinal portal. The limbs of the pericardial cavity can be seen, one on each side, and the cardiogenic mesoderm is found on the ventral aspect of the cavity.
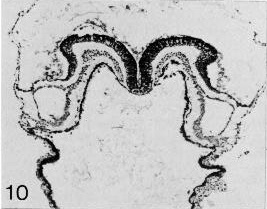
Fig. 9-10. A view of the neural groove and pericardioperitoneal canals in the region of the future midgut.

Fig. 9-11. Transverse section through one of the pairs of somites. So-called myocoeles are visible.

Fig. 9-12. Section through the hindgut.
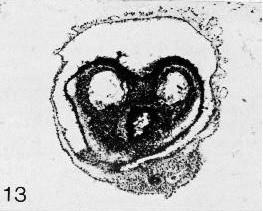
Fig. 9-13. Section through the hindgut and caudally located, bilateral coelomic cavities. The allantoic primordium is visible in cross section in the lowermost portion of the photomicrograph.

Fig. 9-14. Section through the caudal end of the amniotic cavity (lower quarter of photomicrograph) to show the chorion and the chorionic villi (upper two-thirds of photomicrograph).
Page 92SPECIMENS OF STAGE 9 ALREADY DESCRIBED
1 somite, Carnegie No. 5080. Studied and illustrated by Davis (1927, figs. 2–5 and 39–42) and Severn (1971, figs. 1–4). First pair of somites not separate rostrally and contain no myocoeles (Arey, 1938). Chorion, 14.5 x 1.5 mm. Embryonic disc, 1.5 mm. Reconstructed by Müller and O'Rahilly (1983, fig. 2).
1 somite. A specimen described briefly by Baginski and Borsuk (1967).
1–2 (or more?) somites, Carnegie No. 7650. Reconstructed by Müller and O'Rahilly (1983, fig. 5).
2 somites, Da 1 (Dann). An important specimen (fig. 9-2) possessing 2 pairs of somites (Studnicka, 1929; Florian and Völker, 1929; Arey, 1938), although featured originally as having only one. Described and illustrated in detail by Ludwig (1928). Removed from uterus. Chorion, 12 mm. Embryo, 1.8 mm in a straight line, 2.4 mm by flexible scale. Sectioned transversely at 8 μm. Stained with alum cochineal. Neurenteric canal present. Sections are housed in the Anatomisches Institut, Basel. Photographs of sections are in Carnegie Collection under No. 5982. Presumed age, about 21 days. Dorsal and median projections published (ibid., figs. 1 and 2; Florian and Völker, 1929, fig. 14). Reconstructed by Müller and O’Rahilly (1983, fig. 3).
2–3 somites, H3. Described by Wilson (1914), according to whom it “possessed probably two, possibly three, pairs of somites.” Chorion, 8.5 x 5.7 x 5 mm. Embryo, 1.43 mm. Sectioned obliquely (transversely) at 10 μm. Stained with hematoxylin. Fixation not adequate for reconstruction. The relatively longer primitive streak suggests that this embryo may be less advanced than No. 1878. Prechordal plate, or at least prechordal mesoderm, figured (Hill and Florian, 1931b). Presumed age, 18–21 days.
2/3 somites, Carnegie No. 1878 (figs. 9-3 to 9-7). An important specimen possessing 2 somites on the right side and 3 on the left. Florian (1934b) had certain difficulties and considered the embryo to be too small. Curettage. Chorion, 12 X 10.5 X 7.5 mm. Embryonic disc, 1.38 mm in a straight line. Described in detail and illustrated by Ingalls (1920) who believed that “the earliest recognizable stage of dextrocardia” is present, “to which might have been added later a more or less complete situs inversus viscerum”; at any rate, Davis (1927), who studied and illustrated the heart, considered that “the cardiac area is distorted.” Angiogenesis in chorion described by Hertig (1935). Primitive streak and node, 0.13 mm, according to Ingalls, but about 0.22 mm in fig. 15 of Florian and Völker (1929) and more than 0.3 mm in plate 5, fig. 9, of Bartelmez and Evans (1926). Neurenteric canal not patent but pit present (Bartelmez and Evans, 1926). Median projection published (ibid., plate 5, fig. 9; Florian and Völker, 1929, fig. 15; Müller and O’Rahilly, 1983, fig. 1).
3 somites, T439 (Toronto). Possesses 3 pairs of somites (Arey, 1938), although considered originally as having only 2. Described by Piersol (1939). Embryo (along surface), 2.03 x 0.72 mm. Sectioned sagitally. Neurenteric canal closed but its remains are identifiable. Primordial germ cells near allantois. Embryonic disc rostral to somites, including cardiac area, is retarded. Said to contain no blood vessels in any part of the embryo itself.
3 somites, Vant embryo. Described by Shaner (1945), who found “two to three pairs of somites.” Embryo (along curve), 1.5 mm. Thought to be 25 ± 2 days. Reconstructed again from original sections by Müller and O’Rahilly (1983, fig. 4).
3 somites, Gv (Madrid). Described by Jiménez Collado and Ruano Gil (1963). Heart described by Orts Llorca, Jiménez Collado, and Ruano Gil (1960). Tubal. Embryo, 1.81 mm. Sectioned at 7 μm. Stained with hematoxylin and eosin. Reconstructed. On the basis of its external characters, said to lie between stage 9 and stage 10. Presumed age, 21 ± 1 days.
3 somites, No. 2008 (Prague). Excellent specimen (figs. 9-8 to 9-14) belonging to Dr. J. E. Jirásek. Embryo, 1.73 mm. Fixed in calcium formol. Sectioned transversely at 10 μm. Various stains used, including histochemical procedures. Should be published.
3 somites (?), His embryo E. This 2.1-mm specimen is listed by Bartelmez and Evans (1926) between No. 1878 (2–3 somitic pairs) and No. 3709 (4 somitic pairs, stage 10).
Copyright © 1987 Carnegie Institution of Washington. Reproduced on ehd.org with permission.
